Recent Developments in mRNA-Based Protein Supplementation Therapy to Target Lung Diseases
- PMID: 30905577
- PMCID: PMC6453549
- DOI: 10.1016/j.ymthe.2019.02.019
Recent Developments in mRNA-Based Protein Supplementation Therapy to Target Lung Diseases
Abstract
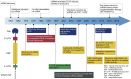
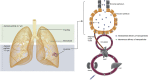
Protein supplementation therapy using in vitro-transcribed (IVT) mRNA for genetic diseases contains huge potential as a new class of therapy. From the early ages of synthetic mRNA discovery, a great number of studies showed the versatile use of IVT mRNA as a novel approach to supplement faulty or absent protein and also as a vaccine. Many modifications have been made to produce high expressions of mRNA causing less immunogenicity and more stability. Recent advancements in the in vivo lung delivery of mRNA complexed with various carriers encouraged the whole mRNA community to tackle various genetic lung diseases. This review gives a comprehensive overview of cells associated with various lung diseases and recent advancements in mRNA-based protein replacement therapy. This review also covers a brief summary of developments in mRNA modifications and nanocarriers toward clinical translation.
Keywords: in vitro transcription; lung; mRNA; nanotransporter; protein supplement.
Copyright © 2019. Published by Elsevier Inc.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

