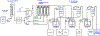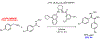Continuous Platform to Generate Nitroalkanes On-Demand (in situ) using Peracetic Acid-Mediated Oxidation in a PFA Pipes-in-Series Reactor
- PMID: 30906182
- PMCID: PMC6424520
- DOI: 10.1021/acs.oprd.8b00113
Continuous Platform to Generate Nitroalkanes On-Demand (in situ) using Peracetic Acid-Mediated Oxidation in a PFA Pipes-in-Series Reactor
Abstract
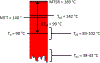
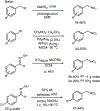
The synthetic utility of the aza-Henry reaction can be diminished on scale by potential hazards associated with the use of peracid to prepare nitroalkane substrates, and the nitroalkanes themselves. In response, a continuous and scalable chemistry platform to prepare aliphatic nitroalkanes on-demand is reported, using the oxidation of oximes with peracetic acid and direct reaction of the nitroalkane intermediate in an aza-Henry reaction. A uniquely designed pipes-in-series plug flow tube reactor addresses a range of process challenges including stability and safe handling of peroxides and nitroalkanes. The subsequent continuous extraction generates a solution of purified nitroalkane which can be directly used in the following enantioselective aza-Henry chemistry to furnish valuable chiral diamine precursors in high selectivity, thus, completely avoiding isolation of potentially unsafe low molecular weight nitroalkane intermediate. A continuous campaign (16 h) established that these conditions were effective in processing 100 g of the oxime and furnishing 1.4 L of nitroalkane solution.
Keywords: continuous processing; flow chemistry; nitroalkane; peroxide; pipes-in-series reactor; sustainable or green chemistry.
Conflict of interest statement
Notes The authors declare no competing financial interest.
Figures
References
-
- Ono N in The Nitro Group in Organic Synthesis John Wiley & Sons, Inc., New York, USA, 2001.
- Feuer H, Nielsen H, A. T. Eds. Nitro Compounds: Recent Advances in Synthesis and Chemistry; VCH Publishers: New York, 1990.
-
- Rezazadeh S; Devannah V; Watson DA Nickel-Catalyzed C-Alkylation of Nitroalkanes with Unactivated Alkyl Iodides, J. Am. Chem. Soc 2017, 139, 8110. - PMC - PubMed
- Hughes DL Patent Review of Manufacturing Routes to Recently Approved PARP Inhibitors: Olaparib, Rucaparib, and Niraparib, Org. Proc. Res. Dev 2017, 21, 1227.
-
- Noble A; Anderson JC Nitro-Mannich Reaction, Chem. Rev 2013, 113, 2887. - PubMed
-
- Moreau B; Charette AB Expedient Synthesis of Cyclopropane α-Amino Acids by the Catalytic Asymmetric Cyclopropanation of Alkenes Using Iodonium Ylides Derived from Methyl Nitroacetate, J. Am. Chem. Soc 2005, 127, 18014. - PubMed
- Li H; Wang Y; Tang L; Wu FH; Liu XF; Guo CY; Foxman BM; Deng L Stereocontrolled Creation of Adjacent Quaternary and Tertiary Stereocenters by a Catalytic Conjugate Addition, Angew. Chem., Int. Ed. 2004, 44, 105. - PubMed
- Knudsen KR; Jorgensen KA A chiral molecular recognition approach to the formation of optically active quaternary centres in aza-Henry reactions, Org. Biomol. Chem 2005, 3, 1362. - PubMed
- (d) Shen B; Johnston JN A Formal Enantioselective Acetate Mannich Reaction: The Nitro Functional Group as a Traceless Agent for Activation and Enantiocontrol in the Synthesis of β-Amino Acids, Org. Lett 2008, 10, 4397. - PubMed
-
- Wilt JC; Pink M; Johnston JN A diastereo- and enantioselective synthesis of α-substituted anti-α,β-diaminophosphonic acid derivatives, Chem. Commun 2008, 35, 4177. - PubMed
- Blaszczyk R; Gajda A; Zawadzki S; Czubacka E; Gajda T N-Carbamate α-aminoalkyl-p-tolylsulfones—convenient substrates in the nitro-Mannich synthesis of secondary N-carbamate protected syn-2-amino-1-nitroalkanephosphonates, Tetrahedron 2010, 66, 9840.
Grants and funding
LinkOut - more resources
Full Text Sources