Viral quasispecies of hepatitis B virus in patients with YMDD mutation and lamivudine resistance may not predict the efficacy of lamivudine/adefovir rescue therapy
- PMID: 30906435
- PMCID: PMC6425149
- DOI: 10.3892/etm.2019.7255
Viral quasispecies of hepatitis B virus in patients with YMDD mutation and lamivudine resistance may not predict the efficacy of lamivudine/adefovir rescue therapy
Abstract
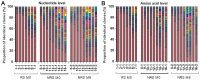
The association between hepatitis B virus (HBV) quasispecies (QS) and the efficacy of nucleos(t)ide analog therapy is currently not well defined, particularly in the case of lamivudine (LAM)/adefovir (ADV) combination rescue therapy for patients with chronic HBV infection (CHB) presenting with LAM resistance. In the present study, 16 CHB patients with the rtM204I/V mutation in the tyrosine-methionine-aspartate-aspartate motif of the C domain of the polymerase gene who switched to LAM/ADV treatment due to LAM resistance were assessed. HBV DNA was isolated from these patients and the reverse transcriptase (RT) region was sequenced. The QS heterogeneity and distribution was analyzed, the mutation sites were recorded and the phylogenetic trees were constructed. The results indicated that QS heterogeneity and distribution in the RT and S regions were not significantly different between responders (RS) and non-RS (NRS) at baseline (P>0.05), except for the higher frequency of a dominant strain in the RT region at the nucleotide level in the RS group (P=0.039). In addition, in NRS, no significant difference in QS heterogeneity or distribution in these regions was identified at six months vs. the baseline. Furthermore, although in the non-responder group the frequency of the LAM resistance-associated mutations (rtM204V/I) decreased at 6 months compared with the baseline, it did not disappear in any of the patients after six months of treatment. Analysis of individual patients did not indicate any consistent selection of specific HBV mutants during LAM/ADV rescue therapy. In conclusion, the baseline HBV QS within the RT and S regions may not be a valid predictor of the response to LAM/ADV rescue treatment in CHB patients with LAM resistance.
Keywords: adefovir; hepatitis B virus; lamivudine; quasispecies; resistance mutation.
Figures




References
-
- Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI. Rescue therapy for lamivudine-resistant chronic hepatitis B: Comparison between entecavir 1.0 mg monotherapy, adefovir monotherapy and adefovir add-on lamivudine combination therapy. J Gastroenterol Hepatol. 2010;25:1374–1380. doi: 10.1111/j.1440-1746.2010.06381.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
