Autologous bone marrow stem cell therapy for patients undergoing coronary artery bypass grafting: A meta-analysis of 14 randomized controlled trials
- PMID: 30906476
- PMCID: PMC6425237
- DOI: 10.3892/etm.2019.7283
Autologous bone marrow stem cell therapy for patients undergoing coronary artery bypass grafting: A meta-analysis of 14 randomized controlled trials
Abstract
Autologous bone marrow stem cell (BMSC) therapy is a novel option for regenerative therapy in patients with ischemic heart disease. The aim of the present meta-analysis was to evaluate the effectiveness of BMSCs combined with coronary artery bypass grafting (CABG). The PubMed, Cochrane Library, EMBASE and Web of Science databases were searched from inception to November 22, 2017 for randomized controlled trials on BMSC therapy combined with CABG. Finally, 14 trials with a total of 596 participants were included. Data were analyzed using a random-effects model. Compared with the control group, the BMSC therapy group exhibited an improvement in the left ventricular (LV) ejection fraction from baseline to follow-up [mean difference (MD)=4.36%; 95% confidence interval (CI): 1.90-6.81%; P<0.01]. Analysis of the pooled results revealed non-significant differences in the LV end-diastolic volume (MD=-6.27 ml; 95% CI: -22.34 to 9.80 ml; P=0.44), LV end-diastolic volume index (MD=-15.11 ml/m2; 95% CI: -31.53 to 1.30 ml/m2; P=0.07), LV end-systolic volume (MD=-11.52 ml; 95% CI: -26.97 to 3.93 ml; P=0.14) and LV end-systolic volume index (MD=-16.56 ml/m2; 95% CI: -37.75 to 4.63 ml/m2; P=0.13) between the BMSC and CABG alone groups. Therefore, autologous BMSC therapy for patients undergoing CABG appears to be associated with an improvement in LV function compared with CABG alone.
Keywords: bone marrow; coronary artery bypass; meta-analysis; randomized controlled trial; stem cell.
Figures

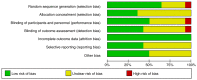





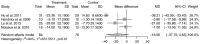

References
-
- Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, et al. Heart Disease and Stroke Statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–e360. - PubMed
-
- Noiseux N, Mansour S, Weisel R, Stevens LM, Der Sarkissian S, Tsang K, Crean AM, Larose E, Li SH, Wintersperger B, et al. The IMPACT-CABG trial: A multicenter, randomized clinical trial of CD133+ stem cell therapy during coronary artery bypass grafting for ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2016;152:1582–1588. doi: 10.1016/j.jtcvs.2016.07.067. - DOI - PubMed
-
- Hu S, Liu S, Zheng Z, Yuan X, Li L, Lu M, Shen R, Duan F, Zhang X, Li J, et al. Isolated coronary artery bypass graft combined with bone marrow mononuclear cells delivered through a graft vessel for patients with previous myocardial infarction and chronic heart failure: A single-center, randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol. 2011;57:2409–2415. doi: 10.1016/j.jacc.2011.01.037. - DOI - PubMed
LinkOut - more resources
Full Text Sources
