Synthesis and Characterization of 3-(1-((3,4-Dihydroxyphenethyl)amino)ethylidene)-chroman-2,4-dione as a Potential Antitumor Agent
- PMID: 30906500
- PMCID: PMC6393868
- DOI: 10.1155/2019/2069250
Synthesis and Characterization of 3-(1-((3,4-Dihydroxyphenethyl)amino)ethylidene)-chroman-2,4-dione as a Potential Antitumor Agent
Abstract
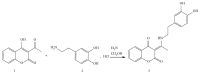
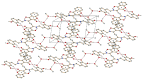
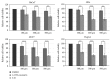
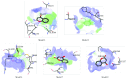
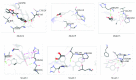
The newly synthesized coumarin derivative with dopamine, 3-(1-((3,4-dihydroxyphenethyl)amino)ethylidene)-chroman-2,4-dione, was completely structurally characterized by X-ray crystallography. It was shown that several types of hydrogen bonds are present, which additionally stabilize the structure. The compound was tested in vitro against different cell lines, healthy human keratinocyte HaCaT, cervical squamous cell carcinoma SiHa, breast carcinoma MCF7, and hepatocellular carcinoma HepG2. Compared to control, the new derivate showed a stronger effect on both healthy and carcinoma cell lines, with the most prominent effect on the breast carcinoma MCF7 cell line. The molecular docking study, obtained for ten different conformations of the new compound, showed its inhibitory nature against CDKS protein. Lower inhibition constant, relative to one of 4-OH-coumarine, proved stronger and more numerous interactions with CDKS protein. These interactions were carefully examined for both parent molecule and derivative and explained from a structural point of view.
Figures

References
-
- Rosselli S., Maggio A. M., Faraone N., et al. The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelinol. Natural Product Communications. 2009;4(12):1701–1706. - PubMed
-
- Atta-ur-Rahman, Shabbir M., Ziauddin Sultani S., Jabbar A., Iqbal Choudhary M. Cinnamates and coumarins from the leaves of Murraya paniculata . Phytochemistry. 1997;44(4):683–685. doi: 10.1016/S0031-9422(96)00617-6. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

