Relation of Redox and Structural Alterations of Rat Skin in the Function of Chronological Aging
- PMID: 30906501
- PMCID: PMC6393874
- DOI: 10.1155/2019/2471312
Relation of Redox and Structural Alterations of Rat Skin in the Function of Chronological Aging
Abstract
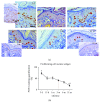
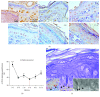
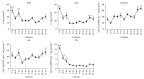
Accumulation of oxidative insults on molecular and supramolecular levels could compromise renewal potency and architecture in the aging skin. To examine and compare morphological and ultrastructural changes with redox alterations during chronological skin aging, activities of antioxidant defense (AD) enzymes, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione reductase (GR), thioredoxin reductase (TR), and methionine sulfoxide reductase A (MsrA), and the markers of oxidative damage of biomolecules-4-hydroxynonenal (HNE) and 8-oxoguanine (8-oxoG)-were examined in the rat skin during life (from 3 days to 21 months). As compared to adult 3-month-old skin, higher activities of CAT, GSH-Px, and GR and a decline in expression of MsrA are found in 21-month-old skin. These changes correspond to degenerative changes at structural and ultrastructural levels in epidermal and dermal compartments, low proliferation capacity, and higher levels of HNE-modified protein aldehydes (particularly in basal lamina) and 8-oxoG positivity in nuclei and mitochondria in the sebaceous glands and root sheath. In 3-day-old skin, higher activities of AD enzymes (SOD, CAT, GR, and TR) and MsrA expression correspond to intensive postnatal development and proliferation. In contrast to 21-month-old skin, a high level of HNE in young skin is not accompanied by 8-oxoG positivity or any morphological disturbances. Observed results indicate that increased activity of AD enzymes in elderly rat skin represents the compensatory response to accumulated oxidative damage of DNA and proteins, accompanied by attenuated repair and proliferative capacity, but in young rats the redox changes are necessary and inherent with processes which occur during postnatal skin development. Мorphological and ultrastructurаl changes are in line with the redox profile in the skin of young and old rats.
Figures




References
-
- Yoshimura H. Organ system in adaptation: the skin. In: Wilber C. G., editor. Handbook of Physiology. Washington, D.C.: American Physiological Society; 1963. pp. 109–133.
-
- Michelson A. M. Liposomal SOD and its application to skin diseases and other disorders. Japanese Journal of Dermatology. 1986;96:1376–1383.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

