The Helicobacter pylori HopQ outermembrane protein inhibits immune cell activities
- PMID: 30906650
- PMCID: PMC6422397
- DOI: 10.1080/2162402X.2018.1553487
The Helicobacter pylori HopQ outermembrane protein inhibits immune cell activities
Abstract
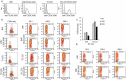
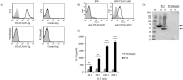
We previously showed that the colorectal cancer colonizing bacterium Fusobacterium nucleatum protects tumors from immune cell attack via binding of the fusbacterial Fap2 outer-membrane protein to TIGIT, a checkpoint inhibitory receptor expressed on T cells and NK cells. Helicobacter pylori, the causative agent for peptic ulcer disease, is associated with the development of gastric adenocarcinoma and MALT lymphoma. The HopQ outer-membrane adhesin of H. pylori was recently shown to bind carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) including CEACAM1, an inhibitory receptor expressed mainly by activated T and NK cells. Here we investigated the possibility that similar to Fap2, HopQ can also inhibit immune cell activities by interacting with CEACAM1. We used several approaches to confirm that HopQ indeed interacts with CEACAM1, and show that CEACAM1-mediated activation by HopQ, may inhibit NK and T cell functions.
Keywords: CEACAM1; Helicobacter pylori; immune cells.
Figures


References
-
- Mc CW, Mason JM 3rd.. Enterococcal endocarditis associated with carcinoma of the sigmoid; report of a case. J Med Assoc State Ala. 1951;21:162–166. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
