Making cold malignant pleural effusions hot: driving novel immunotherapies
- PMID: 30906651
- PMCID: PMC6422374
- DOI: 10.1080/2162402X.2018.1554969
Making cold malignant pleural effusions hot: driving novel immunotherapies
Abstract
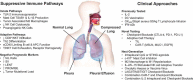
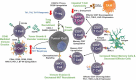
Malignant pleural effusions, arising from either primary mesotheliomas or secondary malignancies, heralds advanced disease and poor prognosis. Current treatments, including therapeutic thoracentesis and tube thoracostomy, are largely palliative. The immunosuppressive environment within the pleural cavity includes myeloid derived suppressor cells, T-regulatory cells, and dysfunctional T cells. The advent of effective immunotherapy with checkpoint inhibitors and adoptive cell therapies for lung cancer and other malignancies suggests a renewed examination of local and systemic therapies for this malady. Prior strategies reporting remarkable success, including instillation of the cytokine interleukin-2, perhaps coupled with checkpoint inhibitors, should be further evaluated in the modern era.
Keywords: Malignant pleural effusion (MPE); adoptive cell therapy (ACT); cancer immunotherapies; damage associated molecular pattern molecules (DAMPs); interleukin-2 (IL-2); mesothelioma (MM); non-small cell lung cancer (NSCLC).
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
