Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma
- PMID: 30906667
- PMCID: PMC6422393
- DOI: 10.1080/2162402X.2019.1571388
Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma
Abstract
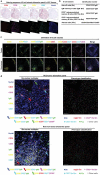
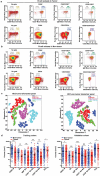
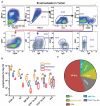
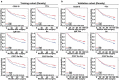
As a major cellular component in tumor microenvironment, the distribution, frequency, and prognostic significance of infiltrating B cell subsets in hepatocellular carcinoma (HCC) remain controversial. Using tyramide signal amplification (TSA) based fluorescent multiplexed immunohistochemistry in situ, we evaluated the distribution and frequency of B cell subsets in two independent HCC cohorts (n = 619). The results were further confirmed by flow cytometry. Correlations of B cell subsets with clinicopathologic features and patient prognosis were analyzed. Five B cell subsets were defined by multiplexed immunohistochemistry and each subset was clearly separated by t-SNE dimension reduction analysis. Notably, the densities of all B cell subsets were significantly decreased in the tumor. The frequency of plasma cells within B cells was most abundant in the tumor. In training cohort (n = 258), high densities of tumor-infiltrating CD20+ B cells, naive B cells, IgM+ memory B cells, CD27- isotype-switched memory B cells, and plasma cells were associated with superior survival. Multivariate analysis further identified CD20+ B cells, naive B cells, and CD27- isotype-switched memory B cells as independent prognosticators for survival. Unsupervised cluster analysis confirmed increased B cell subsets harbored superior survival. In addition, high density of B cells was correlated with smaller tumor size and well differentiation. The results were validated in the independent cohort of 361 HCC patients. Intratumor infiltration of B cells is significantly impaired during HCC progression. High densities of tumor-infiltrating B cells imply a better clinical outcome. Therapies designed to target B cells may be a novel strategy in HCC.
Keywords: B cell subsets; Liver cancer; prognosis; tumor microenvironment (TME); tyramide signal amplification (TSA).
Figures
Similar articles
-
Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma.Clin Cancer Res. 2013 Nov 1;19(21):5994-6005. doi: 10.1158/1078-0432.CCR-12-3497. Epub 2013 Sep 20. Clin Cancer Res. 2013. PMID: 24056784
-
Liver-infiltrating CD11b-CD27- NK subsets account for NK-cell dysfunction in patients with hepatocellular carcinoma and are associated with tumor progression.Cell Mol Immunol. 2017 Oct;14(10):819-829. doi: 10.1038/cmi.2016.28. Epub 2016 Jun 20. Cell Mol Immunol. 2017. PMID: 27321064 Free PMC article.
-
Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma.Gut. 2017 Feb;66(2):342-351. doi: 10.1136/gutjnl-2015-310814. Epub 2015 Dec 15. Gut. 2017. PMID: 26669617 Free PMC article.
-
Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer.Clin Cancer Res. 2018 Dec 15;24(24):6125-6135. doi: 10.1158/1078-0432.CCR-18-1481. Epub 2018 Jul 26. Clin Cancer Res. 2018. PMID: 30049748 Review.
-
Clinicopathologic and prognostic significance of tumor-infiltrating CD8+ T cells in patients with hepatocellular carcinoma: A meta-analysis.Medicine (Baltimore). 2019 Jan;98(2):e13923. doi: 10.1097/MD.0000000000013923. Medicine (Baltimore). 2019. PMID: 30633166 Free PMC article. Review.
Cited by
-
The Emerging Role of B Cells in the Pathogenesis of NAFLD.Hepatology. 2021 Oct;74(4):2277-2286. doi: 10.1002/hep.31889. Epub 2021 Jun 18. Hepatology. 2021. PMID: 33961302 Free PMC article. Review.
-
B cells in pancreatic cancer stroma.World J Gastroenterol. 2022 Mar 21;28(11):1088-1101. doi: 10.3748/wjg.v28.i11.1088. World J Gastroenterol. 2022. PMID: 35431504 Free PMC article. Review.
-
The Role of TGF-β in Bone Metastases.Biomolecules. 2021 Nov 6;11(11):1643. doi: 10.3390/biom11111643. Biomolecules. 2021. PMID: 34827641 Free PMC article. Review.
-
T-cell exhaustion signatures characterize the immune landscape and predict HCC prognosis via integrating single-cell RNA-seq and bulk RNA-sequencing.Front Immunol. 2023 Mar 15;14:1137025. doi: 10.3389/fimmu.2023.1137025. eCollection 2023. Front Immunol. 2023. PMID: 37006257 Free PMC article.
-
Hypoxia-related prognostic model in bladder urothelial reflects immune cell infiltration.Am J Cancer Res. 2021 Oct 15;11(10):5076-5093. eCollection 2021. Am J Cancer Res. 2021. PMID: 34765313 Free PMC article.
References
-
- El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim T.Y., Choo S.P., Trojan J., Welling T.H. 3rd, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi:10.1016/S0140-6736(17)31046-2. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
