Translational inhibition of α-synuclein by Posiphen normalizes distal colon motility in transgenic Parkinson mice
- PMID: 30906671
- PMCID: PMC6420700
Translational inhibition of α-synuclein by Posiphen normalizes distal colon motility in transgenic Parkinson mice
Abstract
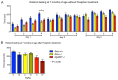
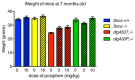
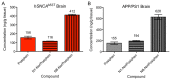
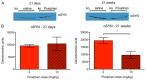
Parkinson disease (PD) is a neurodegenerative disease with motor as well as non-motor symptoms, including gastrointestinal dysfunction. In humans, these precede the motor symptoms by decades. Previously developed and characterized transgenic mice expressing the mutant human α-synuclein gene (SNCA) (either A53T or A30P), but not the endogenous mouse Snca, serve as models for familial PD. These animals demonstrate both robust abnormalities in enteric nervous system (ENS) function as well as synuclein-immunoreactive aggregates in ENS ganglia by 3 months of age, recapitulating early gastrointestinal abnormalities seen before the gait impairment characteristics of human and murine PD. Posiphen is a translational inhibitor of α-synuclein that targets the 5' untranslated region (UTR) of SNCA mRNA and could be a potential drug for the treatment of PD. However, its efficacy in ameliorating symptoms of PD has not yet been evaluated. Here, we used these transgenic mouse models to investigate the efficacy of Posiphen in reversing the gastrointestinal dysfunction. We show that Posiphen normalizes the colonic motility of both transgenic mouse models, although it did not affect the Whole Gut Transit Time (WGTT). Pharmacokinetics studies revealed that Posiphen is more abundant in the brain than in blood, in agreement with its lipophilicity, and the main metabolite is N8-NorPosiphen, a molecule with similar properties as Posiphen. The brain Posiphen levels necessary to effect optimal function were calculated and compared with efficacious brain levels from previous studies, showing that a 2-3 mM concentration of Posiphen and metabolites is sufficient for functional efficacy. Finally, 10 mg/kg Posiphen reduced α-synuclein levels in the gut of hSNCAA53T mice treated for twenty-one weeks, while 50 and 65 mg/kg Posiphen reduced α-synuclein levels in the brain of hSNCAA53T mice treated for twenty-one days. In conclusion, this is the first study showing the preclinical efficacy of Posiphen in normalizing the colonic motility in mouse models of gastrointestinal dysfunction in early PD. This result is in agreement with the ability of Posiphen to reach the nervous system, and its mechanism of action, the translational inhibition of α-synuclein expression. These significant findings support further development of Posiphen as a drug for the treatment of PD.
Keywords: Parkinson’s disease; Posiphen; colonic motility; gastrointestinal dysfunction; α-synuclein.
Conflict of interest statement
None.
Figures




Similar articles
-
Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes.Hum Mol Genet. 2010 May 1;19(9):1633-50. doi: 10.1093/hmg/ddq038. Epub 2010 Jan 27. Hum Mol Genet. 2010. PMID: 20106867 Free PMC article.
-
Genomic DNA levels of mutant alpha-synuclein correlate with non-motor symptoms in an A53T Parkinson's disease mouse model.Neurochem Int. 2018 Mar;114:71-79. doi: 10.1016/j.neuint.2018.01.006. Epub 2018 Jan 23. Neurochem Int. 2018. PMID: 29355568
-
ATH434 Reverses Colorectal Dysfunction in the A53T Mouse Model of Parkinson's Disease.J Parkinsons Dis. 2021;11(4):1821-1832. doi: 10.3233/JPD-212731. J Parkinsons Dis. 2021. PMID: 34366375 Free PMC article.
-
Gastric motor dysfunctions in Parkinson's disease: Current pre-clinical evidence.Parkinsonism Relat Disord. 2015 Dec;21(12):1407-14. doi: 10.1016/j.parkreldis.2015.10.011. Epub 2015 Oct 21. Parkinsonism Relat Disord. 2015. PMID: 26499757 Review.
-
Prion-like propagation of α-synuclein in the gut-brain axis.Brain Res Bull. 2018 Jun;140:341-346. doi: 10.1016/j.brainresbull.2018.06.002. Epub 2018 Jun 9. Brain Res Bull. 2018. PMID: 29894766 Review.
Cited by
-
Relationship among Parkinson's disease, constipation, microbes, and microbiological therapy.World J Gastroenterol. 2024 Jan 21;30(3):225-237. doi: 10.3748/wjg.v30.i3.225. World J Gastroenterol. 2024. PMID: 38314132 Free PMC article. Review.
-
Iron-Mediated Overexpression of Amyloid Precursor Protein via Iron Responsive mRNA in Alzheimer's Disease.Int J Mol Sci. 2025 May 30;26(11):5283. doi: 10.3390/ijms26115283. Int J Mol Sci. 2025. PMID: 40508094 Free PMC article. Review.
-
Novel approaches to counter protein aggregation pathology in Parkinson's disease.Prog Brain Res. 2020;252:451-492. doi: 10.1016/bs.pbr.2019.10.007. Epub 2019 Nov 30. Prog Brain Res. 2020. PMID: 32247372 Free PMC article. Review.
-
New Pieces for an Old Puzzle: Approaching Parkinson's Disease from Translatable Animal Models, Gut Microbiota Modulation, and Lipidomics.Nutrients. 2023 Jun 16;15(12):2775. doi: 10.3390/nu15122775. Nutrients. 2023. PMID: 37375679 Free PMC article. Review.
-
Novel Therapeutic Horizons: SNCA Targeting in Parkinson's Disease.Biomolecules. 2024 Aug 6;14(8):949. doi: 10.3390/biom14080949. Biomolecules. 2024. PMID: 39199337 Free PMC article. Review.
References
-
- Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003;348:1356–1364. - PubMed
-
- Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249(Suppl 3) III/1-5. - PubMed
-
- Halliday GM, Del Tredici K, Braak H. Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson’s disease. J Neural Transm Suppl. 2006:99–103. - PubMed
-
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. - PubMed
-
- Wolters EC, Braak H. Parkinson’s disease: premotor clinico-pathological correlations. J Neural Transm Suppl. 2006:309–319. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
