Drug Delivery From Polymer-Based Nanopharmaceuticals-An Experimental Study Complemented by Simulations of Selected Diffusion Processes
- PMID: 30906737
- PMCID: PMC6418005
- DOI: 10.3389/fbioe.2019.00037
Drug Delivery From Polymer-Based Nanopharmaceuticals-An Experimental Study Complemented by Simulations of Selected Diffusion Processes
Abstract
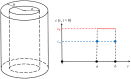
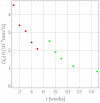
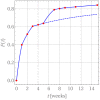
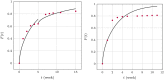
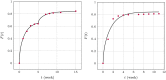
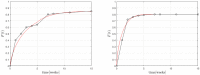
The success of medical therapy depends on the correct amount and the appropriate delivery of the required drugs for treatment. By using biodegradable polymers a drug delivery over a time span of weeks or even months is made possible. This opens up a variety of strategies for better medication. The drug is embedded in a biodegradable polymer (the "carrier") and injected in a particular position of the human body. As a consequence of the interplay between the diffusion process and the degrading polymer the drug is released in a controlled manner. In this work we study the controlled release of medication experimentally by measuring the delivered amount of drug within a cylindrical shell over a long time interval into the body fluid. Moreover, a simple continuum model of the Fickean type is initially proposed and solved in closed-form. It is used for simulating some of the observed release processes for this type of carrier and takes the geometry of the drug container explicitly into account. By comparing the measurement data and the model predictions diffusion coefficients are obtained. It turns out that within this simple model the coefficients change over time. This contradicts the idea that diffusion coefficients are constants independent of the considered geometry. The model is therefore extended by taking an additional absorption term into account leading to a concentration dependent diffusion coefficient. This could now be used for further predictions of drug release in carriers of different shape. For a better understanding of the complex diffusion and degradation phenomena the underlying physics is discussed in detail and even more sophisticated models involving different degradation and mass transport phenomena are proposed for future work and study.
Keywords: biphosphonate; diffusion coefficient; gentamicin; modeling; polylacetic acid.
Figures





Similar articles
-
Model-based optimization of drug release rate from a size distributed population of biodegradable polymer carriers.Eur J Pharm Biopharm. 2023 May;186:112-131. doi: 10.1016/j.ejpb.2023.02.014. Epub 2023 Mar 2. Eur J Pharm Biopharm. 2023. PMID: 36870398
-
Controlled diffusional release of dispersed solute drugs from biodegradable implants of various geometries.Biomed Sci Instrum. 1997;33:137-42. Biomed Sci Instrum. 1997. PMID: 9731349
-
Transport Properties for Pharmaceutical Controlled-Release Systems: A Brief Review of the Importance of Their Study in Biological Systems.Biomolecules. 2018 Dec 17;8(4):178. doi: 10.3390/biom8040178. Biomolecules. 2018. PMID: 30563024 Free PMC article. Review.
-
Mathematical modeling of PLGA microparticles: from polymer degradation to drug release.Mol Pharm. 2014 Nov 3;11(11):4036-48. doi: 10.1021/mp500078u. Epub 2014 Oct 2. Mol Pharm. 2014. PMID: 25230105
-
Mathematical modeling of bioerodible, polymeric drug delivery systems.Adv Drug Deliv Rev. 2001 Jun 11;48(2-3):229-47. doi: 10.1016/s0169-409x(01)00116-8. Adv Drug Deliv Rev. 2001. PMID: 11369084 Review.
Cited by
-
Delivery of TGFβ3 from Magnetically Responsive Coaxial Fibers Reduces Spinal Cord Astrocyte Reactivity In Vitro.Adv Biol (Weinh). 2024 Oct;8(10):e2300531. doi: 10.1002/adbi.202300531. Epub 2024 Jun 27. Adv Biol (Weinh). 2024. PMID: 38935534
-
The synthesis and development of poly(ε-caprolactone) conjugated polyoxyethylene sorbitan oleate-based micelles for curcumin drug release: an in vitro study on breast cancer cells.RSC Adv. 2023 Aug 4;13(34):23449-23460. doi: 10.1039/d3ra03660j. eCollection 2023 Aug 4. RSC Adv. 2023. PMID: 37546220 Free PMC article.
-
Titanium membrane layered between fluvastatin-loaded poly (lactic-co-glycolic) acid for guided bone regeneration.Regen Biomater. 2022 Sep 6;9:rbac061. doi: 10.1093/rb/rbac061. eCollection 2022. Regen Biomater. 2022. PMID: 36237949 Free PMC article.
-
A novel injectable fibromodulin-releasing granular hydrogel for tendon healing and functional recovery.Bioeng Transl Med. 2022 Jul 14;8(1):e10355. doi: 10.1002/btm2.10355. eCollection 2023 Jan. Bioeng Transl Med. 2022. PMID: 36684085 Free PMC article.
-
Activated Carbon-Enriched Electrospun-Produced Scaffolds for Drug Delivery/Release in Biological Systems.Int J Mol Sci. 2023 Apr 4;24(7):6713. doi: 10.3390/ijms24076713. Int J Mol Sci. 2023. PMID: 37047685 Free PMC article.
References
-
- (2009). Sink Condition. Available online at: https://en.wikipedia.org/wiki/Sink_condition (Accessed February 5, 2019).
-
- Ben-Nissan B. (2003). Natural bioceramics: from coral to bone and beyond. Curr. Opin. Solid State Mater. Sci. 7, 283–288. 10.1016/j.cossms.2003.10.001 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

