2'-Fucosyllactose Is Well Tolerated in a 100% Whey, Partially Hydrolyzed Infant Formula With Bifidobacterium lactis: A Randomized Controlled Trial
- PMID: 30906817
- PMCID: PMC6421602
- DOI: 10.1177/2333794X19833995
2'-Fucosyllactose Is Well Tolerated in a 100% Whey, Partially Hydrolyzed Infant Formula With Bifidobacterium lactis: A Randomized Controlled Trial
Abstract
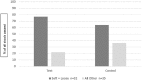
Human milk oligosaccharides are important components of breast milk. We evaluated feeding tolerance of the human milk oligosaccharide 2'-fucosyllactose (2'FL) in a 100% whey, partially hydrolyzed infant formula with the probiotic Bifidobacterium animalis ssp lactis strain Bb12 (B lactis; Test) as compared with the same formula without 2'FL (Control) in a randomized controlled trial of healthy infants enrolled at 2 weeks of age (±5 days). After 6 weeks of feeding the assigned formula, the primary outcome of tolerance was assessed using the Infant Gastrointestinal Symptom Questionnaire. Stooling, vomiting, spit-up, crying, and fussing were compared between groups. Seventy-nine infants were enrolled and 63 completed the study per protocol (30 Test, 33 Control). Infant Gastrointestinal Symptom Questionnaire scores were similar between groups (Test 20.9 ± 4.8, Control 20.7 ± 4.3, P = .82). Partially hydrolyzed infant formula with 2'FL and B lactis is tolerated well, as confirmed by a validated multi-symptom index.
Keywords: 2′-fucosyllactose; gastrointestinal tolerance; human milk oligosaccharides; infant formula; partially hydrolyzed whey.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HMS, LMC, BK, and RC are employees of Nestlé Nutrition, the sponsor of this study. JS, SSC, and HR are paid consultants for Nestlé Nutrition.
Figures
Similar articles
-
Effects of addition of 2-fucosyllactose to infant formula on growth and specific pathways of utilization by Bifidobacterium in healthy term infants.Front Nutr. 2022 Sep 23;9:961526. doi: 10.3389/fnut.2022.961526. eCollection 2022. Front Nutr. 2022. PMID: 36211486 Free PMC article.
-
Safety and efficacy of a probiotic-containing infant formula supplemented with 2'-fucosyllactose: a double-blind randomized controlled trial.Nutr J. 2022 Feb 22;21(1):11. doi: 10.1186/s12937-022-00764-2. Nutr J. 2022. PMID: 35193609 Free PMC article. Clinical Trial.
-
Infants Fed a Lower Calorie Formula With 2'FL Show Growth and 2'FL Uptake Like Breast-Fed Infants.J Pediatr Gastroenterol Nutr. 2015 Dec;61(6):649-58. doi: 10.1097/MPG.0000000000000889. J Pediatr Gastroenterol Nutr. 2015. PMID: 26154029 Free PMC article. Clinical Trial.
-
Review of the Clinical Experiences of Feeding Infants Formula Containing the Human Milk Oligosaccharide 2'-Fucosyllactose.Nutrients. 2018 Sep 21;10(10):1346. doi: 10.3390/nu10101346. Nutrients. 2018. PMID: 30241407 Free PMC article. Review.
-
Milk protein-based infant formula containing rice starch and low lactose reduces common regurgitation in healthy term infants: a randomized, blinded, and prospective trial.J Am Coll Nutr. 2014;33(2):136-46. doi: 10.1080/07315724.2013.828578. J Am Coll Nutr. 2014. PMID: 24724771 Review.
Cited by
-
Infant formulas with synthetic oligosaccharides and respective marketing practices.Mol Cell Pediatr. 2022 Jul 13;9(1):14. doi: 10.1186/s40348-022-00146-y. Mol Cell Pediatr. 2022. PMID: 35831686 Free PMC article.
-
Effects of addition of 2-fucosyllactose to infant formula on growth and specific pathways of utilization by Bifidobacterium in healthy term infants.Front Nutr. 2022 Sep 23;9:961526. doi: 10.3389/fnut.2022.961526. eCollection 2022. Front Nutr. 2022. PMID: 36211486 Free PMC article.
-
Infant Formula With a Specific Blend of Five Human Milk Oligosaccharides Drives the Gut Microbiota Development and Improves Gut Maturation Markers: A Randomized Controlled Trial.Front Nutr. 2022 Jul 6;9:920362. doi: 10.3389/fnut.2022.920362. eCollection 2022. Front Nutr. 2022. PMID: 35873420 Free PMC article.
-
Safety and Tolerance of a Novel Anti-Regurgitation Formula: A Double-Blind, Randomized, Controlled Trial.J Pediatr Gastroenterol Nutr. 2021 Nov 1;73(5):579-585. doi: 10.1097/MPG.0000000000003289. J Pediatr Gastroenterol Nutr. 2021. PMID: 34417399 Free PMC article. Clinical Trial.
-
Lactational and geographical variation in the concentration of six oligosaccharides in Chinese breast milk: a multicenter study over 13 months postpartum.Front Nutr. 2023 Sep 5;10:1267287. doi: 10.3389/fnut.2023.1267287. eCollection 2023. Front Nutr. 2023. PMID: 37731395 Free PMC article.
References
-
- Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A, Gabrielli O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr Suppl. 1999;88:89-94. - PubMed
-
- Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699-722. - PubMed
-
- Castanys-Muñoz E, Martin M, Prieto P. 2′-Fucosyllactose: an abundant, genetically determined soluble glycan present in human milk. Nutr Rev. 2013;71:773-789. - PubMed
LinkOut - more resources
Full Text Sources
Medical