Impact of sex on pain and opioid analgesia: a review
- PMID: 30906823
- PMCID: PMC6428208
- DOI: 10.1016/j.cobeha.2018.08.001
Impact of sex on pain and opioid analgesia: a review
Abstract
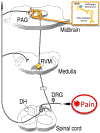
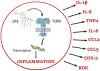
Chronic pain is a debilitating condition that impacts tens of millions each year, resulting in lost wages for workers and exacting considerable costs in health care and rehabilitation. A thorough understanding of the neural mechanisms underlying pain and analgesia is critical to facilitate the development of therapeutic strategies and personalized medicine. Clinical and epidemiological studies report that women experience greater levels of pain than men and have higher rates of pain-related disorders. Studies in both rodents and humans report sex differences in the anatomical and physiologic properties of the descending antinociceptive circuit, mu opioid receptor (MOR) expression and binding, morphine metabolism, and immune system activation, all of which likely contribute to the observed sex differences in pain and opioid analgesia. Although more research is needed to elucidate the underlying mechanisms, these sex differences present potential therapeutic targets to optimize pain management strategies for both sexes.
Figures
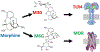

Similar articles
-
Sex Differences in Microglia Activity within the Periaqueductal Gray of the Rat: A Potential Mechanism Driving the Dimorphic Effects of Morphine.J Neurosci. 2017 Mar 22;37(12):3202-3214. doi: 10.1523/JNEUROSCI.2906-16.2017. Epub 2017 Feb 20. J Neurosci. 2017. PMID: 28219988 Free PMC article.
-
Heteromerization of μ-opioid receptor and cholecystokinin B receptor through the third transmembrane domain of the μ-opioid receptor contributes to the anti-opioid effects of cholecystokinin octapeptide.Exp Mol Med. 2018 May 21;50(5):1-16. doi: 10.1038/s12276-018-0090-5. Exp Mol Med. 2018. PMID: 29780163 Free PMC article.
-
[Development of opioid tolerance -- molecular mechanisms and clinical consequences].Anasthesiol Intensivmed Notfallmed Schmerzther. 2003 Jan;38(1):14-26. doi: 10.1055/s-2003-36558. Anasthesiol Intensivmed Notfallmed Schmerzther. 2003. PMID: 12522725 Review. German.
-
T394A Mutation at the μ Opioid Receptor Blocks Opioid Tolerance and Increases Vulnerability to Heroin Self-Administration in Mice.J Neurosci. 2016 Oct 5;36(40):10392-10403. doi: 10.1523/JNEUROSCI.0603-16.2016. J Neurosci. 2016. PMID: 27707973 Free PMC article.
-
Opioids in chronic pain.Eur J Pharmacol. 2001 Oct 19;429(1-3):79-91. doi: 10.1016/s0014-2999(01)01308-5. Eur J Pharmacol. 2001. PMID: 11698029 Review.
Cited by
-
Darting across space and time: parametric modulators of sex-biased conditioned fear responses.Learn Mem. 2022 Jun 16;29(7):171-180. doi: 10.1101/lm.053587.122. Print 2022 Jul. Learn Mem. 2022. PMID: 35710304 Free PMC article.
-
Integration of primary contact physiotherapists in the emergency department for individuals presenting with minor musculoskeletal disorders: Protocol for an economic evaluation.PLoS One. 2023 Sep 14;18(9):e0277369. doi: 10.1371/journal.pone.0277369. eCollection 2023. PLoS One. 2023. PMID: 37708179 Free PMC article.
-
The role of gonadal hormones in regulating opioid antinociception.Ann Med. 2024 Dec;56(1):2329259. doi: 10.1080/07853890.2024.2329259. Epub 2024 May 13. Ann Med. 2024. PMID: 38738380 Free PMC article. Review.
-
Does heightened subjective sexual arousal lower pain in women if disgust is minimized?PLoS One. 2025 May 20;20(5):e0323095. doi: 10.1371/journal.pone.0323095. eCollection 2025. PLoS One. 2025. PMID: 40392787 Free PMC article.
-
Morphine-context associative memory and locomotor sensitization in mice are modulated by sex and context in a dose-dependent manner.bioRxiv [Preprint]. 2023 Nov 5:2023.11.03.565492. doi: 10.1101/2023.11.03.565492. bioRxiv. 2023. PMID: 37961152 Free PMC article. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
