pH-dependent gating mechanism of the Helicobacter pylori urea channel revealed by cryo-EM
- PMID: 30906870
- PMCID: PMC6426461
- DOI: 10.1126/sciadv.aav8423
pH-dependent gating mechanism of the Helicobacter pylori urea channel revealed by cryo-EM
Abstract
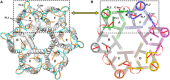
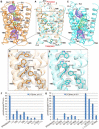
The urea channel of Helicobacter pylori (HpUreI) is an ideal drug target for preventing gastric cancer but incomplete understanding of its gating mechanism has hampered development of inhibitors for the eradication of H. pylori. Here, we present the cryo-EM structures of HpUreI in closed and open conformations, both at a resolution of 2.7 Å. Our hexameric structures of this small membrane protein (~21 kDa/protomer) resolve its periplasmic loops and carboxyl terminus that close and open the channel, and define a gating mechanism that is pH dependent and requires cooperativity between protomers in the hexamer. Gating is further associated with well-resolved changes in the channel-lining residues that modify the shape and length of the urea pore. Site-specific mutations in the periplasmic domain and urea pore identified key residues important for channel function. Drugs blocking the urea pore based on our structures should lead to a new strategy for H. pylori eradication.
Figures


References
-
- Hooi J. K. Y., Lai W. Y., Ng W. K., Suen M. M. Y., Underwood F. E., Tanyingoh D., Malfertheiner P., Graham D. Y., Wong V. W. S., Wu J. C. Y., Chan F. K. L., Sung J. J. Y., Kaplan G. G., Ng S. C., Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 153, 420–429 (2017). - PubMed
-
- Herrera V., Parsonnet J., Helicobacter pylori and gastric adenocarcinoma. Clin. Microbiol. Infect. 15, 971–976 (2009). - PubMed
-
- Jung D. H., Kim J.-H., Lee Y. C., Lee S. K., Shin S. K., Park J. C., Chung H. S., Kim H., Kim H., Kim Y. H., Park J. J., Youn Y. H., Park H., Helicobacter pylori eradication reduces the metachronous recurrence of gastric neoplasms by attenuating the precancerous process. J. Gastric Cancer 15, 246–255 (2015). - PMC - PubMed
-
- Choi I. J., Kook M.-C., Kim Y.-I., Cho S.-J., Lee J. Y., Kim C. G., Park B., Nam B.-H., Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N. Engl. J. Med. 378, 1085–1095 (2018). - PubMed

