Development and Validation of an Immunoassay for Tenofovir in Urine as a Real-Time Metric of Antiretroviral Adherence
- PMID: 30906930
- PMCID: PMC6428441
- DOI: 10.1016/j.eclinm.2018.08.004
Development and Validation of an Immunoassay for Tenofovir in Urine as a Real-Time Metric of Antiretroviral Adherence
Abstract
Background: Pharmacologic adherence measures were critical to the interpretation of the tenofovir (TFV)-disoproxil-fumarate/emtricitabine (TDF/FTC) PrEP trials. These measures are being incorporated into PrEP demonstration projects, but currently-available metrics in plasma, cells, hair or urine involve expensive and time-intensive mass-spectrometry (MS)-based methods. No point-of-care method to assess PrEP adherence in real-time has yet been implemented. Antibody-based tests allow for low-cost, easy-to-perform, point-of-care drug detection. In this study, we developed an antibody-based TFV immunoassay and evaluated its test characteristics among individuals taking TDF/FTC.
Methods: We synthesized possible immunogens based on TFV's molecular structure, injected rabbits with the conjugated derivatives, and bled them monthly for subsequent ELISA-testing for TFV-specific antibodies. We purified an antibody with specific TFV binding and created dose-response curves for ELISA-quantification. We then quantified TFV in urine from human participants not taking TDF/FTC and from individuals taking daily TDF/FTC 300 mg/200 mg for 7 days with a 7-day washout period using ELISA with this TFV-specific antibody. ELISA results were compared with the gold-standard test for TFV detection/quantification using liquid-chromatography-tandem-MS (LC-MS/MS).
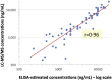
Findings: None of the urine samples from 115 participants not taking TDF/FTC showed ELISA- reactivity, indicating 100% specificity (95% CI 97-100%) of the immunoassay. Among participants taking TDF/FTC, 67 of 70 samples positive by LC-MS/MS were positive by the ELISA-immunoassay for an estimated diagnostic sensitivity of 96% (95% CI 88-99%). The precision of the assay was high (coefficient of variationb15%). The rank correlation between ELISA and LC-MS/MS values in the 70 quantitative urine TFV levels positive by LC-MS/MS across a wide range of concentrations among participants on TDF/FTC was high (r = 0.96).
Interpretation: Our antibody-based immunoassay for measuring TFV in urine performed well compared to the gold-standard of LC-MS/MS among individuals taking TDF/FTC. A sensitive and specific immunoassay paves the way for real-time monitoring/feedback on recent adherence to TFV-based regimens, which should optimize interpretation and outcomes during PrEP and ART roll-out.
Keywords: Antibody; Antiretroviral adherence; Antiretroviral treatment; Immunoassay; Point-of-care; PrEP; Real-time; Tenofovir; Test characteristics; Urine.
Conflict of interest statement
Conflict of Interest None of the authors have any conflicts of interests to report.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
