Direct capture and conversion of CO2 from air by growing a cyanobacterial consortium at pH up to 11.2
- PMID: 30906982
- PMCID: PMC6593468
- DOI: 10.1002/bit.26974
Direct capture and conversion of CO2 from air by growing a cyanobacterial consortium at pH up to 11.2
Abstract
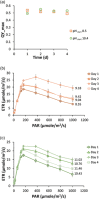
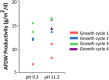
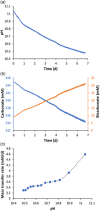
Bioenergy with carbon capture and storage (BECCS) is recognized as a potential negative emission technology, needed to keep global warming within safe limits. With current technologies, large-scale implementation of BECCS would compromise food production. Bioenergy derived from phototrophic microorganisms, with direct capture of CO2 from air, could overcome this challenge and become a sustainable way to realize BECCS. Here we present an alkaline capture and conversion system that combines high atmospheric CO2 transfer rates with high and robust phototrophic biomass productivity (15.2 ± 1.0 g/m 2 /d). The system is based on a cyanobacterial consortium, that grows at high alkalinity (0.5 mol/L) and a pH swing between 10.4 and 11.2 during growth and harvest cycles.
Keywords: BECCS; Cyanobacteria consortium; alkalinity; direct carbon capture; photosynthesis; soda Lakes.
© 2019 The Authors. Biotechnology and Bioengineering Published by Wiley Periodicals, Inc.
Figures


References
-
- Beal, C. M. , Archibald, I. , Huntley, M. E. , Greene, C. H. , & Johnson, Z. I. (2018). Integrating algae with bioenergy carbon capture and storage (ABECCS) increases sustainability. Earth's Future, 6(3), 524–542. 10.1002/2017EF000704 - DOI
-
- Beardall, J. , & Raven, J. A. (1981). Transport of inorganic carbon and the ‘CO2concentrating mechanism’ in Chlorella Emersonii (Chlorophyceae). Journal of Phycology, 17, 134–141. 10.1111/j.1529-8817.1981.tb00832.x - DOI
-
- Bui, M. , Adjiman, C. S. , Bardow, A. , Anthony, E. J. , Boston, A. , Brown, S. , & Mac Dowell, N. (2018). Carbon capture and storage (CCS): The way forward. Energy and Environmental Science, 11(5), 1062–1176. 10.1039/c7ee02342a - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

