Sal B targets TAZ to facilitate osteogenesis and reduce adipogenesis through MEK-ERK pathway
- PMID: 30907511
- PMCID: PMC6484321
- DOI: 10.1111/jcmm.14272
Sal B targets TAZ to facilitate osteogenesis and reduce adipogenesis through MEK-ERK pathway
Abstract
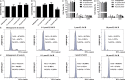
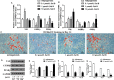
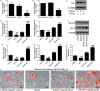
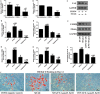
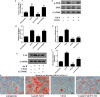
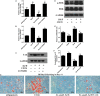
Salvianolic acid B (Sal B), a major bioactive component of Chinese herb, was identified as a mediator for bone metabolism recently. The aim of this study is to investigate the underlying mechanisms by which Sal B regulates osteogenesis and adipogenesis. We used MC3T3-E1 and 3T3-L1 as the study model to explore the changes of cell differentiation induced by Sal B. The results indicated that Sal B at different concentrations had no obvious toxicity effects on cell proliferation during differentiation. Furthermore, Sal B facilitated osteogenesis but inhibited adipogenesis by increasing the expression of transcriptional co-activator with PDZ-binding motif (TAZ). Accordingly, TAZ knock-down offset the effects of Sal B on cell differentiation into osteoblasts or adipocytes. Notably, the Sal B induced up-expression of TAZ was blocked by U0126 (the MEK-ERK inhibitor), rather than LY294002 (the PI3K-Akt inhibitor). Moreover, Sal B increased the p-ERK/ERK ratio to regulate the TAZ expression as well as the cell differentiation. In summary, this study suggests for the first time that Sal B targets TAZ to facilitate osteogenesis and reduce adipogenesis by activating MEK-ERK signalling pathway, which provides evidence for Sal B to be used as a potential therapeutic agent for the management of bone diseases.
Keywords: MEK-ERK pathway; Salvianolic acid B; TAZ; adipogenesis; osteogenesis.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors confirm that there is no conflict of interests.
Figures

Similar articles
-
Salvianolic acid B promotes osteogenesis of human mesenchymal stem cells through activating ERK signaling pathway.Int J Biochem Cell Biol. 2014 Jun;51:1-9. doi: 10.1016/j.biocel.2014.03.005. Epub 2014 Mar 19. Int J Biochem Cell Biol. 2014. PMID: 24657587
-
FGF2 stimulates osteogenic differentiation through ERK induced TAZ expression.Bone. 2014 Jan;58:72-80. doi: 10.1016/j.bone.2013.09.024. Epub 2013 Oct 11. Bone. 2014. PMID: 24125755
-
TM-25659 enhances osteogenic differentiation and suppresses adipogenic differentiation by modulating the transcriptional co-activator TAZ.Br J Pharmacol. 2012 Mar;165(5):1584-94. doi: 10.1111/j.1476-5381.2011.01664.x. Br J Pharmacol. 2012. PMID: 21913895 Free PMC article.
-
Salvianolic acid B prevents bone loss in prednisone-treated rats through stimulation of osteogenesis and bone marrow angiogenesis.PLoS One. 2012;7(4):e34647. doi: 10.1371/journal.pone.0034647. Epub 2012 Apr 6. PLoS One. 2012. PMID: 22493705 Free PMC article.
-
Effect and mechanism of psoralidin on promoting osteogenesis and inhibiting adipogenesis.Phytomedicine. 2019 Aug;61:152860. doi: 10.1016/j.phymed.2019.152860. Epub 2019 Feb 4. Phytomedicine. 2019. PMID: 31048126
Cited by
-
TAZ promotes osteogenic differentiation of mesenchymal stem cells line C3H10T1/2, murine multi-lineage cells lines C2C12, and MEFs induced by BMP9.Cell Death Discov. 2022 Dec 27;8(1):499. doi: 10.1038/s41420-022-01292-y. Cell Death Discov. 2022. PMID: 36575168 Free PMC article.
-
Curculigoside Ameliorates Bone Loss by Influencing Mesenchymal Stem Cell Fate in Aging Mice.Front Cell Dev Biol. 2021 Dec 3;9:767006. doi: 10.3389/fcell.2021.767006. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34926455 Free PMC article.
-
Salvia miltiorrhiza in osteoporosis: a review of its phytochemistry, traditional clinical uses and preclinical studies (2014-2024).Front Pharmacol. 2024 Oct 3;15:1483431. doi: 10.3389/fphar.2024.1483431. eCollection 2024. Front Pharmacol. 2024. PMID: 39421672 Free PMC article. Review.
-
Myostatin suppresses adipogenic differentiation and lipid accumulation by activating crosstalk between ERK1/2 and PKA signaling pathways in porcine subcutaneous preadipocytes.J Anim Sci. 2021 Dec 1;99(12):skab287. doi: 10.1093/jas/skab287. J Anim Sci. 2021. PMID: 34634123 Free PMC article.
-
Glucocorticoids decreased Cx43 expression in osteonecrosis of femoral head: The effect on proliferation and osteogenic differentiation of rat BMSCs.J Cell Mol Med. 2021 Jan;25(1):484-498. doi: 10.1111/jcmm.16103. Epub 2020 Nov 17. J Cell Mol Med. 2021. PMID: 33205619 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

