Mechanistic Dichotomy in Proton-Coupled Electron-Transfer Reactions of Phenols with a Copper Superoxide Complex
- PMID: 30907590
- PMCID: PMC6584633
- DOI: 10.1021/jacs.9b00466
Mechanistic Dichotomy in Proton-Coupled Electron-Transfer Reactions of Phenols with a Copper Superoxide Complex
Abstract
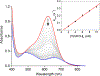
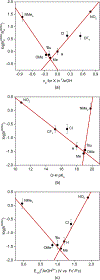
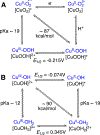
The kinetics and mechanism(s) of the reactions of [K(Krypt)][LCuO2] (Krypt = 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane, L = a bis(arylcarboxamido)pyridine ligand) with 2,2,6,6-tetramethylpiperdine- N-hydroxide (TEMPOH) and the para-substituted phenols XArOH (X = para substituent NO2, CF3, Cl, H, Me, tBu, OMe, or NMe2) at low temperatures were studied. The reaction with TEMPOH occurs rapidly ( k = 35.4 ± 0.3 M-1 s-1) by second-order kinetics to yield TEMPO• and [LCuOOH]- on the basis of electron paramagnetic resonance spectroscopy, the production of H2O2 upon treatment with protic acid, and independent preparation from reaction of [NBu4][LCuOH] with H2O2 ( Keq = 0.022 ± 0.007 for the reverse reaction). The reactions with XArOH also follow second-order kinetics, and analysis of the variation of the k values as a function of phenol properties (Hammett σ parameter, O-H bond dissociation free energy, p Ka, E1/2) revealed a change in mechanism across the series, from proton transfer/electron transfer for X = NO2, CF3, Cl to concerted-proton/electron transfer (or hydrogen-atom transfer) for X = OMe, NMe2 (data for X = H, Me, tBu are intermediate between the extremes). Thermodynamic analysis and comparisons to previous results for LCuOH, a different copper-oxygen intermediate with the same supporting ligand, and literature for other [CuO2]+ complexes reveal significant differences in proton-coupled electron-transfer mechanisms that have implications for understanding oxidation catalysis by copper-containing enzymes and abiological catalysts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Selected reviews: Solomon EI; Heppner DE; Johnston EM; Ginsbach JW; Cirera J; Qayyum M; Kieber-Emmons MT; Kjaergaard CH; Hadt RG; Tian L Copper Active Sites in Biology. Chem. Rev 2014, 114, 3659–3853. - PMC - PubMed
- Vu VV; Ngo ST Copper active site in polysaccharide monooxygenases. Coord. Chem. Rev 2018, 368, 134–157.
- Ciano L; Davies GJ; Tolman WB; Walton PH Bracing copper for the catalytic oxidation of C−H bonds. Nature Catal 2018, 1, 571–577.
- Quist DA; Diaz DE; Liu JJ; Karlin KD Activation of dioxygen by copper metalloproteins and insights from model complexes. JBIC, J. Biol. Inorg. Chem 2017, 22, 253–288. - PMC - PubMed
- Tandrup T; Frandsen KEH; Johansen KS; Berrin JG; Lo Leggio L Recent insights into lytic polysaccharide monooxygenases (LPMOs). Biochem. Soc. Trans 2018, 46, 1431–1447. - PubMed
-
- Selected lead references: Trammell R; See YY; Herrmann AT; Xie N; Diaz DE; Siegler MA; Baran PS; Garcia-Bosch I Decoding the Mechanism of Intramolecular Cu-Directed Hydroxlation of sp3 C-H Bonds. J. Org. Chem 2017, 82, 7887–7904. - PMC - PubMed
- Snyder BER; Bols ML; Schoonheydt RA; Sels BF; Solomon EI Iron and Copper Active Sites in Zeolites and Their Correlation to Metalloenzymes. Chem. Rev 2018, 118, 2718–2768. - PubMed
- Pegis ML; Wise CF; Martin DJ; Mayer JM Oxygen Reduction by Homogeneous Molecular Catalysts and Electrocatalysts. Chem. Rev 2018, 118, 2340–2391. - PubMed
- Gandeepan P; Müller T; Zell D; Cera G; Warratz S; Ackermann L 3d Transition Metals for C−H Activation. Chem. Rev 2019, 119, 2192–2452. - PubMed
-
- Hedegård ED; Ryde U Targeting the reactive intermediate in polysaccharide monooxygenases. JBIC, J. Biol. Inorg. Chem 2017, 22, 1029–1037. - PMC - PubMed
- Hedegård ED; Ryde U Molecular mechanism of lytic polysaccharide monooxygenases. Chem. Sci 2018, 9, 3866–3880. - PMC - PubMed
- Caldararu O; Oksanen E; Ryde U; Hedegård ED Mechanism of hydrogen peroxide formation by lytic polysaccharide monooxygenase. Chem. Sci 2019, 10, 576–586. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

