Off-label prescriptions of drugs used for the treatment of Crohn's disease or ulcerative colitis
- PMID: 30908719
- PMCID: PMC6593662
- DOI: 10.1111/apt.15229
Off-label prescriptions of drugs used for the treatment of Crohn's disease or ulcerative colitis
Abstract
Background: Off-label prescribing is encountered across various fields of medicine and creates alternative treatment options, but is associated with unknown safety risks. The use of off-label drugs for the treatment of patients with inflammatory bowel diseases (IBD) has not been characterised before.
Aim: To assess the proportion and characteristics of off-label prescribing for IBD in tertiary care centres in the Netherlands.
Methods: A prospective database of IBD patients from all Dutch university hospitals was used to collect data on drug prescriptions for IBD and demographics. Drugs were classified as off-label if they were unlicensed for Crohn's disease and/or ulcerative colitis by the Medicines Evaluation Board. Uni- and multivariable analyses were used to identify patient-specific characteristics predictive of increased off-label use.
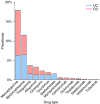
Results: For the induction and/or maintenance treatment of 4583 IBD patients, 12 651 historical and current drug records were available in the database. Of these, 2374 (19%) were considered off-label prescriptions. Out of 4583 IBD patients, 1477 (32%) were exposed to off-label drugs. Commonly prescribed off-label IBD drugs were mercaptopurine (18%), beclomethasone (12%), thioguanine (4%) and allopurinol (3%). Non-thiopurine/methotrexate off-label drugs were prescribed in 243 patients (6%), including biological agents or tofacitinib in 47 IBD patients (1%). Off-label prescriptions were more common in ulcerative colitis than Crohn's disease (37% vs 29%, P < 0.001). Smokers and patients that received ≥5 drug types during their disease course were more likely to be exposed to off-label drugs (smoking 33% vs 27% and multiple drug use 66% vs 22%, both P < 0.001).
Conclusion: About one-fifth of prescriptions for IBD were off-label and one-third of IBD patients, especially ulcerative colitis patients, were exposed to off-label drugs.
Keywords: Crohn’s disease; drugs; inflammatory bowel disease; off-label; prescriptions; therapeutic care; ulcerative colitis.
© 2019 The Authors. Alimentary Pharmacology & Therapeutics Published by John Wiley & Sons Ltd.
Figures
Comment in
-
Letter: off-label use of hyperbaric oxygen therapy in inflammatory bowel disease-Authors' reply.Aliment Pharmacol Ther. 2020 Jul;52(1):216-217. doi: 10.1111/apt.15806. Aliment Pharmacol Ther. 2020. PMID: 32529768 No abstract available.
-
Letter: off-label use of hyperbaric oxygen therapy in inflammatory bowel disease.Aliment Pharmacol Ther. 2020 Jul;52(1):215-216. doi: 10.1111/apt.15775. Aliment Pharmacol Ther. 2020. PMID: 32529770 No abstract available.
References
-
- Aagaard L, Kristensen K. Off‐label and unlicensed prescribing in Europe: implications for patients' informed consent and liability. Int J Clin Pharm. 2018;40:509‐512. - PubMed
-
- Eguale T, Buckeridge DL, Verma A, et al. Association of off‐label drug use and adverse drug events in an adult population. JAMA Intern Med. 2016;176:55‐63. - PubMed
-
- Administration FaD . Patents and Exclusivity. 2015. https://www.fda.gov/downloads/drugs/developmentapprovalprocess/smallbusi.... Accessed March 2, 2019.
-
- Furey K, Wilkins K. Prescribing, "Off‐Label": What should a physician disclose? AMA J Ethics. 2016;18:587‐593. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
