Impact of Curcumin (with or without Piperine) on the Pharmacokinetics of Tamoxifen
- PMID: 30909366
- PMCID: PMC6468355
- DOI: 10.3390/cancers11030403
Impact of Curcumin (with or without Piperine) on the Pharmacokinetics of Tamoxifen
Abstract
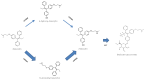
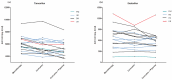
Tamoxifen is a prodrug that is primarily metabolized into the pharmacologically active metabolite endoxifen and eventually into inactive metabolites. The herb curcumin may increase endoxifen exposure by affecting phase II metabolism. We compared endoxifen and tamoxifen exposure in breast cancer patients with or without curcumin, and with addition of the bio-enhancer piperine. Tamoxifen (20⁻30mg per day (q.d.)) was either given alone, or combined with curcumin (1200 mg three times daily (t.i.d.)) +/- piperine (10 mg t.i.d.). The primary endpoint of this study was the difference in geometric means for the area under the curve (AUC) of endoxifen. Genotyping was performed to determine CYP2D6 and CYP3A4 phenotypes. The endoxifen AUC0⁻24h decreased with 7.7% (95%CI: -15.4 to 0.7%; p = 0.07) with curcumin and 12.4% (95%CI: -21.9 to -1.9%; p = 0.02) with curcumin and piperine, compared to tamoxifen alone. Tamoxifen AUC0⁻24h showed similar results. For patients with an extensive CYP2D6 metabolism phenotype (EM), effects were more pronounced than for intermediate CYP2D6 metabolizers (IMs). In conclusion, the exposure to tamoxifen and endoxifen was significantly decreased by concomitant use of curcumin (+/- piperine). Therefore, co-treatment with curcumin could lower endoxifen concentrations below the threshold for efficacy (potentially 20⁻40% of the patients), especially in EM patients.
Keywords: curcumin; drug interactions; pharmacokinetics; piperine; tamoxifen.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. This work was presented as a proffered paper at the 54th ASCO annual meeting (4 June 2018, Chicago, IL, USA, abstract number: 2572).
Figures
References
-
- Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

