Change in Renal Glomerular Collagens and Glomerular Filtration Barrier-Related Proteins in a Dextran Sulfate Sodium-Induced Colitis Mouse Model
- PMID: 30909435
- PMCID: PMC6471354
- DOI: 10.3390/ijms20061458
Change in Renal Glomerular Collagens and Glomerular Filtration Barrier-Related Proteins in a Dextran Sulfate Sodium-Induced Colitis Mouse Model
Abstract
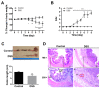
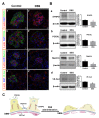
Renal disease is not rare among patients with inflammatory bowel disease (IBD) and is gaining interest as a target of research. However, related changes in glomerular structural have rarely been investigated. This study was aimed at clarifying the changes in collagens and glomerular filtration barrier (GFB)-related proteins of glomeruli in a dextran sulfate sodium (DSS)-induced colitis mouse model. Acute colitis was induced by administering 3.5% DSS in Slc:ICR strain mice for eight days. Histological changes to glomeruli were examined by periodic acid-Schiff (PAS) and Masson's trichrome staining. Expressions of glomerular collagens and GFB-related proteins were analyzed by immunofluorescent staining and Western blot analysis. DSS-colitis mice showed an elevated disease activity index (DAI), colon shortening, massive cellular infiltration and colon damage, confirming that DSS-colitis mice can be used as an IBD animal model. DSS-colitis mice showed increased glycoprotein and collagen deposition in glomeruli. Interestingly, we observed significant changes in glomerular collagens, including a decrease in type IV collagen, and an increment in type I and type V collagens. Moreover, declined GFB-related proteins expressions were detected, including synaptopodin, podocalyxin, nephrin and VE-cadherin. These results suggest that renal disease in DSS-colitis mice might be associated with changes in glomerular collagens and GFB-related proteins. These findings are important for further elucidation of the clinical pathological mechanisms underlying IBD-associated renal disease.
Keywords: DSS-colitis; glomerular filtration barrier (GFB); inflammatory bowel disease (IBD); type I collagen; type IV collagen; type V collagen.
Conflict of interest statement
The authors declare that they have no conflicts of interest, financial or otherwise, regarding this article.
Figures



References
-
- Sartor R.B. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn’s disease. Gastroenterol. Clin. N. Am. 1995;24:475–507. - PubMed
-
- Ricart E., Panaccione R., Loftus E.V., Tremaine W.J., Harmsen W.S., Zinsmeister A.R., Sandborn W.J. Autoimmune disorders and Extraintestinal manifestations in First-degree familial and sporadic inflammatory bowel disease. Inflamm. Bowel Dis. 2004;10:207–214. doi: 10.1097/00054725-200405000-00005. - DOI - PubMed
-
- Christodoulou D.K., Katsanos K.H., Kitsanou M., Stergiopoulou C., Hatzis J., Tsianos E.V. Frequency of extraintestinal manifestations in patients with inflammatory bowel disease in northwest Greece and review of the literature. Dig. Liver Dis. 2002;34:781–786. doi: 10.1016/S1590-8658(02)80071-8. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

