Whole-Cell Multiparameter Assay for Ricin and Abrin Activity-Based Digital Holographic Microscopy
- PMID: 30909438
- PMCID: PMC6468687
- DOI: 10.3390/toxins11030174
Whole-Cell Multiparameter Assay for Ricin and Abrin Activity-Based Digital Holographic Microscopy
Abstract
Ricin and abrin are ribosome-inactivating proteins leading to inhibition of protein synthesis and cell death. These toxins are considered some of the most potent and lethal toxins against which there is no available antidote. Digital holographic microscopy (DHM) is a time-lapse, label-free, and noninvasive imaging technique that can provide phase information on morphological features of cells. In this study, we employed DHM to evaluate the morphological changes of cell lines during ricin and abrin intoxication. We showed that the effect of these toxins is characterized by a decrease in cell confluence and changes in morphological parameters such as cell area, perimeter, irregularity, and roughness. In addition, changes in optical parameters such as phase-shift, optical thickness, and effective-calculated volume were observed. These effects were completely inhibited by specific neutralizing antibodies. An enhanced intoxication effect was observed for preadherent compared to adherent cells, as was detected in early morphology changes and confirmed by annexin V/propidium iodide (PI) apoptosis assay. Detection of the dynamic changes in cell morphology at initial stages of cell intoxication by DHM emphasizes the highly sensitive and rapid nature of this method, allowing the early detection of active toxins.
Keywords: abrin; digital holographic microscopy; holomonitor; intoxication; morphology; ricin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

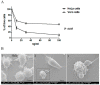



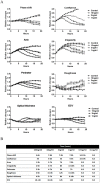
References
-
- Olivieri F., Prasad V., Valbonesi P., Srivastava S., Ghosal-Chowdhury P., Barbieri L., Bolognesi A., Stirpe F. A systemic antiviral resistance-inducing protein isolated from Clerodendrum inerme Gaertn. is a polynucleotide:adenosine glycosidase (ribosome-inactivating protein) FEBS Lett. 1996;396:132–134. doi: 10.1016/0014-5793(96)01089-7. - DOI - PubMed
-
- Endo Y., Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 1987;262:8128–8130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

