Intravenous Immunoglobulin Therapy Eliminates Candida albicans and Maintains Intestinal Homeostasis in a Murine Model of Dextran Sulfate Sodium-Induced Colitis
- PMID: 30909599
- PMCID: PMC6471409
- DOI: 10.3390/ijms20061473
Intravenous Immunoglobulin Therapy Eliminates Candida albicans and Maintains Intestinal Homeostasis in a Murine Model of Dextran Sulfate Sodium-Induced Colitis
Abstract
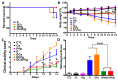
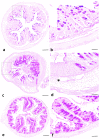
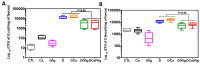
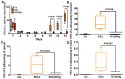
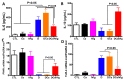
Intravenous immunoglobulin (IVIg) therapy has diverse anti-inflammatory and immunomodulatory effects and has been employed successfully in autoimmune and inflammatory diseases. The role of IVIg therapy in the modulation of intestinal inflammation and fungal elimination has not been yet investigated. We studied IVIg therapy in a murine model of dextran sulfate sodium (DSS)-induced colitis. Mice received a single oral inoculum of Candida albicans and were exposed to DSS treatment for 2 weeks to induce colitis. All mice received daily IVIg therapy starting on day 1 for 7 days. IVIg therapy not only prevented a loss of body weight caused by the development of colitis but also reduced the severity of intestinal inflammation, as determined by clinical and histological scores. IVIg treatment significantly reduced the Escherichia coli, Enterococcus faecalis, and C. albicans populations in mice. The beneficial effects of IVIg were associated with the suppression of inflammatory cytokine interleukin (IL)-6 and enhancement of IL-10 in the gut. IVIg therapy also led to an increased expression of peroxisome proliferator-activated receptor gamma (PPARγ), while toll-like receptor 4 (TLR-4) expression was reduced. IVIg treatment reduces intestinal inflammation in mice and eliminates C. albicans overgrowth from the gut in association with down-regulation of pro-inflammatory mediators combined with up-regulation of anti-inflammatory cytokines.
Keywords: Candida albicans; Enterococcus faecalis; Escherichia coli; colitis; cytokines; dextran sulfate sodium; inflammation; intravenous immunoglobulin G; mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Sendid B., Dotan N., Nseir S., Savaux C., Vandewalle P., Standaert A., Zerimech F., Guery B.P., Dukler A., Colombel J.F., et al. Antibodies against glucan, chitin, and saccharomyces cerevisiae mannan as new biomarkers of candida albicans infection that complement tests based on c. Albicans mannan. Clin. Vaccine Immunol. 2008;15:1868–1877. doi: 10.1128/CVI.00200-08. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

