Preservation of Acyl Coenzyme A Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking
- PMID: 30909726
- PMCID: PMC6557671
- DOI: 10.1161/CIRCULATIONAHA.119.039610
Preservation of Acyl Coenzyme A Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking
Abstract
Background: Metabolic remodeling in heart failure contributes to dysfunctional lipid trafficking and lipotoxicity. Acyl coenzyme A synthetase-1 (ACSL1) facilitates long-chain fatty acid (LCFA) uptake and activation with coenzyme A (CoA), mediating the fate of LCFA. The authors tested whether cardiac ACSL1 overexpression aids LCFA oxidation and reduces lipotoxicity under pathological stress of transverse aortic constriction (TAC).
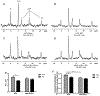
Methods: Mice with cardiac restricted ACSL1 overexpression (MHC-ACSL1) underwent TAC or sham surgery followed by serial in vivo echocardiography for 14 weeks. At the decompensated stage of hypertrophy, isolated hearts were perfused with 13C LCFA during dynamic-mode 13C nuclear magnetic resonance followed by in vitro nuclear magnetic resonance and mass spectrometry analysis to assess intramyocardial lipid trafficking. In parallel, acyl CoA was measured in tissue obtained from heart failure patients pre- and postleft ventricular device implantation plus matched controls.
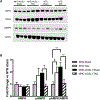
Results: TAC-induced cardiac hypertrophy and dysfunction was mitigated in MHC-ACSL1 hearts compared with nontransgenic hearts. At 14 weeks, TAC increased heart weight to tibia length by 46% in nontransgenic mice, but only 26% in MHC-ACSL1 mice, whereas ACSL1 mice retained greater ejection fraction (ACSL1 TAC: 65.8±7.5%; nontransgenic TAC: 45.9±7.3) and improvement in diastolic E/E'. Functional improvements were mediated by ACSL1 changes to cardiac LCFA trafficking. ACSL1 accelerated LCFA uptake, preventing C16 acyl CoA loss post-TAC. Long-chain acyl CoA was similarly reduced in human failing myocardium and restored to control levels by mechanical unloading. ACSL1 trafficked LCFA into ceramides without normalizing the reduced triglyceride storage in TAC. ACSL1 prevented de novo synthesis of cardiotoxic C16- and C24-, and C24:1 ceramides and increased potentially cardioprotective C20- and C22-ceramides post-TAC. ACLS1 overexpression activated AMP activated protein kinase at baseline, but during TAC, prevented the reduced LCFA oxidation in hypertrophic hearts and normalized energy state (phosphocreatine:ATP) and consequently, AMP activated protein kinase activation.
Conclusions: This is the first demonstration of reduced acyl CoA in failing hearts of humans and mice, and suggests possible mechanisms for maintaining mitochondrial oxidative energy metabolism by restoring long-chain acyl CoA through ASCL1 activation and mechanical unloading. By mitigating cardiac lipotoxicity, via redirected LCFA trafficking to ceramides, and restoring acyl CoA, ACSL1 delayed progressive cardiac remodeling and failure.
Keywords: acyl coenzyme A; ceramides; coenzyme A ligases; fatty acids; heart failure.
Conflict of interest statement
Disclosures
The authors declare no competing interests.
Figures
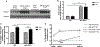
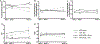



Comment in
-
Letter by Wang et al Regarding Article, "Preservation of Acyl Coenzyme A Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking".Circulation. 2019 Nov 5;140(19):e762-e763. doi: 10.1161/CIRCULATIONAHA.119.042343. Epub 2019 Nov 4. Circulation. 2019. PMID: 31682524 No abstract available.
-
Response by Lewandowski et al to Letter Regarding Article, "Preservation of Acyl Coenzyme A Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking".Circulation. 2019 Nov 5;140(19):e764-e765. doi: 10.1161/CIRCULATIONAHA.119.043152. Epub 2019 Nov 4. Circulation. 2019. PMID: 31682528 Free PMC article. No abstract available.
References
-
- Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation 2007;115:2033–2041. doi: 10.1161/CIRCULATIONAHA.106.668665 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

