Fabrication and Characterization of Electrospun Decellularized Muscle-Derived Scaffolds
- PMID: 30909819
- PMCID: PMC6535957
- DOI: 10.1089/ten.TEC.2018.0339
Fabrication and Characterization of Electrospun Decellularized Muscle-Derived Scaffolds
Abstract
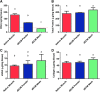
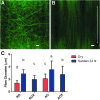
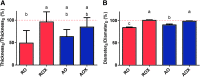
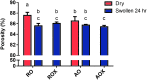
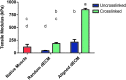
Although skeletal muscle has a high potential for self-repair, volumetric muscle loss can result in impairment beyond the endogenous regenerative capacity. There is a clinical need to improve on current clinical treatments that fail to fully restore the structure and function of lost muscle. Decellularized extracellular matrix (dECM) scaffolds have been an attractive platform for regenerating skeletal muscle, as dECM contains many biochemical cues that aid in cell adhesion, proliferation, and differentiation. However, there is limited capacity to tune physicochemical properties in current dECM technologies to improve outcome. In this study, we aim to create a novel, high-throughput technique to fabricate dECM scaffolds with tunable physicochemical properties while retaining proregenerative matrix components. We demonstrate a successful decellularization protocol that effectively removes DNA. We also identified key steps for the successful production of electrospun muscle dECM without the use of a carrier polymer; electrospinning allows for rapid scaffold fabrication with high control over material properties, which can be optimized to mimic native muscle. To this end, fiber orientation and degree of crosslinking of these dECM scaffolds were modulated and the corollary effects on fiber swelling, mechanical properties, and degradation kinetics were investigated. Beyond application in skeletal muscle, the versatility of this technology has the potential to serve as a foundation for dECM scaffold fabrication in a variety of tissue engineering applications.
Keywords: decellularized muscle; electrospinning; extracellular matrix; skeletal muscle.
Conflict of interest statement
No competing financial interests exist.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

