An observational study in an urban Ugandan clinic comparing virological outcomes of patients switched from first-line antiretroviral regimens to second-line regimens containing ritonavir-boosted atazanavir or ritonavir-boosted lopinavir
- PMID: 30909871
- PMCID: PMC6434787
- DOI: 10.1186/s12879-019-3907-5
An observational study in an urban Ugandan clinic comparing virological outcomes of patients switched from first-line antiretroviral regimens to second-line regimens containing ritonavir-boosted atazanavir or ritonavir-boosted lopinavir
Abstract
Background: The World Health Organisation approved boosted atazanavir as a preferred second line protease inhibitor in 2010. This is as an alternative to the current boosted lopinavir. Atazanavir has a lower genetic barrier than lopinavir. We compared the virological outcomes of patients during the roll out of routine viral load monitoring, who had switched to boosted second- line regimens of either atazanavir or lopinavir.
Methods: This was a cross-sectional study involving adult patients at the Infectious Diseases Institute Kampala, Uganda started on a standard WHO recommended second-line regimen containing either boosted atazanavir or boosted lopinavir between 1 Dec 2014 and 31 July 2015.. Mantel -Haenszel chi square was used to test for the statistical significance of the odds of being suppressed (VL < 400 copies/ml) when on boosted atazanavir compared to boosted lopinavir after stratifying by duration on antiretroviral therapy (ART). Multivariate logistic regression analysis used to determine if the type of boosted protease inhibitor (bPI) was associated with virological outcome.
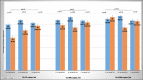
Results: Ninety (90) % on ATV/r and 83% on LPV/r had a VL less than 1000 copies/ml. The odds of being suppressed using the same viral load cut-off while on boosted atazanavir compared to boosted lopinavir was not statistically significant after stratifying for duration on ART (p = 0.09). In a multivariate analysis the type of bPI used was not a predictor of virological outcome (p = 0.60).
Conclusions: Patients using the WHO recommended second-line of boosted atazanavir have comparable virological suppression to those on boosted lopinavir.
Keywords: Atazanavir; First-line failure; Lopinavir; Second-line antiretroviral.
Conflict of interest statement
Ethics approval and consent to participate
All routinely collected clinic data is approved for analysis and reporting by the Makerere University Faculty of Medicine, Research and Ethics committee (approval number: 120–2009) and Uganda National Council for Science and Technology (approval number: 45683) hence the need for consent was waived by an IRB.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Joint United Nations Programme on HIV/AIDS. Global AIDS Update 2016. http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update.... Accessed 10 Jan 2018.
-
- World Health Organisation. Global report: UNAIDS report on the global AIDS epidemic 2013. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Global_Repo.... Accessed 15 Dec 2016.
-
- Somnuek Sungkanuparph WM, 2 Sasisopin Kiertiburanakul,1 Bucha Piyavong,1, Noppanath Chumpathat WC. Options for a Second-Line Antiretroviral Regimen for HIV Type 1–Infected Patients Whose Initial Regimenof a Fixed-Dose Combination of Stavudine,Lamivudine, and Nevirapine Fails. Clin Infect Dis. 2007;44(3):447–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

