Lipopolysaccharide and palmitic acid synergistically induced MCP-1 production via MAPK-meditated TLR4 signaling pathway in RAW264.7 cells
- PMID: 30909920
- PMCID: PMC6434618
- DOI: 10.1186/s12944-019-1017-4
Lipopolysaccharide and palmitic acid synergistically induced MCP-1 production via MAPK-meditated TLR4 signaling pathway in RAW264.7 cells
Abstract
Background: Obesity increases the risk of developing diabetes mellitus. Clinical studies suggest that risk factors like palmitic acid (PA) and lipopolysaccharide (LPS) exist simultaneously in diabetes with obesity. Combination of PA and LPS even at low concentration can induce strong inflammatory reaction. Monocyte chemoattractant protein-1 (MCP-1) is an important inflammatory chemokine related to insulin resistance and type II diabetes. Our previous study using PCR array revealed that LPS and PA synergistically induce MCP-1 mRNA expression in macrophage cells RAW264.7, while the protein expression of MCP-1 in this case was not investigated. Moreover, the underling mechanism in the synergistic effect of MCP-1 expression or production induced by treatment of LPS and PA combination remains unclear.
Methods: Protein secretion of MCP-1 was measured by the enzyme-linked immunosorbent assay (ELISA) and mRNA levels of MCP-1 and Toll-like receptor 4 (TLR4) were measured by real-time PCR. Statistical analysis was conducted using SPSS software.
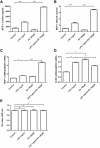
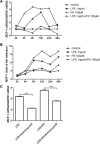
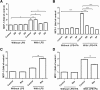
Results: LPS could increase MCP-1 transcription as well as secretion in RAW264.7, and PA amplified this effect obviously. Meanwhile, combination of LPS with PA increased TLR4 mRNA expression while LPS alone or PA alone could not, TLR4 knockdown inhibited MCP-1 transcription/secretion induced by LPS plus PA. Moreover, not NF-κB inhibitor but inhibitors of mitogen-activated protein kinase (MAPK) signaling pathways, including c-Jun NH2-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and p38 MAPK were found to block MCP-1 generation stimulated by LPS plus PA.
Conclusion: LPS and PA synergistically induced MCP-1 secretion in RAW264.7 macrophage cells, in which MCP-1 transcription mediated by MAPK/TLR4 signaling pathways was involved. Combined treatment of PA and LPS in RAW264.7 cells mimics the situation of diabetes with obesity that has higher level of PA and LPS, MAPK/TLR4/ MCP-1 might be potential therapeutic targets for diabetes with obesity.
Keywords: Lipopolysaccharide; MAPK; MCP-1; Palmitic acid; TLR4.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Epigallocatechin-3-Gallate Inhibits Matrix Metalloproteinase-9 and Monocyte Chemotactic Protein-1 Expression Through the 67-κDa Laminin Receptor and the TLR4/MAPK/NF-κB Signalling Pathway in Lipopolysaccharide-Induced Macrophages.Cell Physiol Biochem. 2017;43(3):926-936. doi: 10.1159/000481643. Epub 2017 Sep 29. Cell Physiol Biochem. 2017. PMID: 28957799
-
Anti-Inflammatory Effect of Auranofin on Palmitic Acid and LPS-Induced Inflammatory Response by Modulating TLR4 and NOX4-Mediated NF-κB Signaling Pathway in RAW264.7 Macrophages.Int J Mol Sci. 2021 May 31;22(11):5920. doi: 10.3390/ijms22115920. Int J Mol Sci. 2021. PMID: 34072916 Free PMC article.
-
Acid sphingomyelinase plays a key role in palmitic acid-amplified inflammatory signaling triggered by lipopolysaccharide at low concentrations in macrophages.Am J Physiol Endocrinol Metab. 2013 Oct 1;305(7):E853-67. doi: 10.1152/ajpendo.00251.2013. Epub 2013 Aug 6. Am J Physiol Endocrinol Metab. 2013. PMID: 23921144 Free PMC article.
-
Toll-like receptor 4 signaling is involved in IgA-stimulated mesangial cell activation.Yonsei Med J. 2011 Jul;52(4):610-5. doi: 10.3349/ymj.2011.52.4.610. Yonsei Med J. 2011. PMID: 21623603 Free PMC article.
-
Atorvastatin suppresses LPS-induced rapid upregulation of Toll-like receptor 4 and its signaling pathway in endothelial cells.Am J Physiol Heart Circ Physiol. 2011 May;300(5):H1743-52. doi: 10.1152/ajpheart.01335.2008. Epub 2011 Feb 11. Am J Physiol Heart Circ Physiol. 2011. PMID: 21317307
Cited by
-
Salicylate Sodium Suppresses Monocyte Chemoattractant Protein-1 Production by Directly Inhibiting Phosphodiesterase 3B in TNF-α-Stimulated Adipocytes.Int J Mol Sci. 2022 Dec 24;24(1):320. doi: 10.3390/ijms24010320. Int J Mol Sci. 2022. PMID: 36613764 Free PMC article.
-
MAPKs/AP-1, not NF-κB, is responsible for MCP-1 production in TNF-α-activated adipocytes.Adipocyte. 2022 Dec;11(1):477-486. doi: 10.1080/21623945.2022.2107786. Adipocyte. 2022. PMID: 35941819 Free PMC article.
-
Harnessing the Therapeutic Potential of Ginsenoside Rd for Activating SIRT6 in Treating a Mouse Model of Nonalcoholic Fatty Liver Disease.ACS Omega. 2023 Aug 3;8(32):29735-29745. doi: 10.1021/acsomega.3c04122. eCollection 2023 Aug 15. ACS Omega. 2023. PMID: 37599957 Free PMC article.
-
Gut microbial composition and functionality of school-age Mexican population with metabolic syndrome and type-2 diabetes mellitus using shotgun metagenomic sequencing.Front Pediatr. 2023 May 31;11:1193832. doi: 10.3389/fped.2023.1193832. eCollection 2023. Front Pediatr. 2023. PMID: 37342535 Free PMC article.
-
Anti-inflammatory effect of Angiotensin 1-7 in white adipose tissue.Adipocyte. 2025 Dec;14(1):2449027. doi: 10.1080/21623945.2024.2449027. Epub 2025 Jan 13. Adipocyte. 2025. PMID: 39803918 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

