A new phototherapy regimen during winter as an add-on therapy, coupled with oral vitamin D supplementation, for the long-term control of atopic dermatitis: study protocol for a multicentre, randomized, crossover, pragmatic trial - the PRADA trial
- PMID: 30909923
- PMCID: PMC6434814
- DOI: 10.1186/s13063-019-3276-9
A new phototherapy regimen during winter as an add-on therapy, coupled with oral vitamin D supplementation, for the long-term control of atopic dermatitis: study protocol for a multicentre, randomized, crossover, pragmatic trial - the PRADA trial
Abstract
Background: Atopic dermatitis is a highly prevalent, chronic, relapsing disease in both adults and children. On the severity spectrum, lower-end patients benefit from small amounts of topical anti-inflammatory treatments (TAT), whereas higher-end patients need systemic immunosuppressants; in-between patients are treated with TAT and phototherapy. The major therapeutic challenge in this population is the long-term control of disease activity, and the current TAT-based pro-active strategy does not meet all their needs. Immunosuppressants are used as long-term control add-on treatments, but they are restricted to the most severely affected patients because of safety concerns. In addition, neither immunosuppressants nor other strategies have been properly evaluated in the long term despite long-term control having been acknowledged as one of the most important core outcome domains to be targeted in atopic dermatitis trials. Safe add-on therapies, rigorously evaluated for long-term control of the disease, are therefore needed. Phototherapy and vitamin D supplementation are both good candidates.
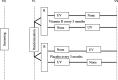
Methods: This is a multicenter, national, randomized, superiority, crossover trial testing add-on phototherapy (one winter under spaced sessions of phototherapy and one winter under observation) among subjects receiving standard care (i.e., TAT). On the same population, we will test the long-term control provided by oral supplementation of vitamin D versus placebo in a randomized, superiority, double-blind, parallel-group trial. The primary outcomes are (1) repeat measures of the PO-SCORAD severity score over 1 year and (2) cumulate consumption of TAT (number of tubes) during the winter. They will be tested following a hierarchical testing procedure. The secondary outcomes will be measures repeated over 2 years of investigator-based severity scores, patient-reported severity and quality of life scores, serum vitamin D levels, weeks during which the disease is well-controlled, inter-visit cumulate consumption of TAT, and synthetic patient-reported satisfaction at the end of each winter.
Discussion: This study includes two separate 2-year pragmatic trials designed to evaluate the efficacy of vitamin D supplementation and pro-active phototherapy for primary care atopic dermatitis patients receiving TAT on long-term control of disease activity. The experimental design enables the study of both interventions and exploration of the interaction between vitamin D and phototherapy. A pragmatic trial is particularly suited to the assessment of long-term control. This study explores the possibility of new and safe therapeutic strategies for the control of long-term atopic dermatitis, and is an example of efficacy research that is unlikely to be sponsored by industrialists. A potentially effective low-cost therapeutic strategy for long-term control is essential for patients and public health.
Trial registration: ClinicalTrials.gov Identifier: NCT02537509 , first received: 1 September 2015.
Keywords: Atopic dermatitis; add-on therapy; long-term control; phototherapy; pragmatic trial; vitamin D.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval for the study was obtained from the institutional review board of the University Hospital Centre of Rennes. Details on the procedures of informed consent are given in the Ethics and dissemination sections.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Williams H, Robertson C, Stewart A, Aït-Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103:125–138. doi: 10.1016/S0091-6749(99)70536-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical