MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma
- PMID: 30909929
- PMCID: PMC6434841
- DOI: 10.1186/s13046-019-1135-x
MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma
Abstract
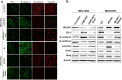
Background: Mitochondrial Ca2+ plays a critical role in tumorigenesis, including cell proliferation and metastasis. Mitochondrial calcium uniporter regulator 1 (MCUR1) has been shown to be frequently upregulated in HCC and promote cancer cell survival. However, whether MCUR1 is involved in the metastasis of HCC and its underlying mechanisms remain unknown.
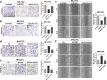
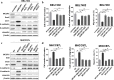
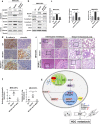
Methods: The effect of MCUR1 expression on epithelial-mesenchymal transition (EMT) in HCC cells was first evaluated by immunofluorescent staining and Western blot. Then, in vitro invasion and in vivo metastasis assays were used to evaluate the function of MCUR1 in HCC metastasis. The underlying mechanism has also been explored by investigating the effect of MCUR1 on ROS/Nrf2/Notch1 pathway.
Results: MCUR1 expression was significantly higher in HCC with metastasis and associated with tumor progression. MCUR1 promoted in vitro invasion and in vivo metastasis of HCC cells by promoting EMT via Snail. Mechanistically, MCUR1-mediated mitochondrial Ca2+ signaling promoted the EMT of HCC cells by activating ROS/Nrf2/Notch1 pathway. Inhibition of ROS production, mitochondrial Ca2+ uptake, Nrf2 expression or Notch1 activity significantly suppressed MCUR1-induced EMT of HCC cells. In addition, treatment with the mitochondrial Ca2+-buffering protein parvalbumin significantly inhibited ROS/Nrf2/Notch pathway and MCUR1-induced EMT and HCC metastasis.
Conclusions: Our study provides evidence supporting a metastasis-promoting role for MCUR1-dependent mitochondrial Ca2+ uptake in HCC. Our findings suggest that MCUR1 may be a potential therapeutic target for HCC treatment.
Keywords: EMT; Hepatocellular carcinoma; Metastasis; Mitochondrial calcium uniporter regulator 1; Notch 1.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Fourth Military Medical University. Animal research was approved by the Institutional Animal Care and Use Committee of Fourth Military Medical University. The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10(2):99–111. doi: 10.1016/j.ccr.2006.06.016. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

