Plant Hsp90 is a novel adjuvant that elicits a strong humoral and cellular immune response against B- and T-cell epitopes of a Toxoplasma gondii SAG1 peptide
- PMID: 30909938
- PMCID: PMC6434815
- DOI: 10.1186/s13071-019-3362-6
Plant Hsp90 is a novel adjuvant that elicits a strong humoral and cellular immune response against B- and T-cell epitopes of a Toxoplasma gondii SAG1 peptide
Abstract
Background: The 90-kDa heat-shock protein (Hsp90) from Nicotiana benthamiana (NbHsp90.3) is a promising adjuvant, especially for those vaccines that require a T cell-mediated immune response. Toxoplasma gondii SAG1 is considered one of the most important antigens for the development of effective subunit vaccines. Some epitopes located in the SAG1 C-terminus region have showed a strong humoral and cellular immune response. In the present study, we aimed to assess the efficacy of NbHsp90.3 as carrier/adjuvant of SAG1-derived peptide (SAG1HC) in a T. gondii infection murine model.
Methods: In the present study, C57BL/6 mice were intraperitoneal immunized with the NbHsp90.3-SAG1HC fusion protein (NbHsp90.3-SAG1HC group), mature SAG1 (SAG1m group), NbHsp90.3 (NbHsp90.3 group) or PBS buffer 1× (PBS group). The levels of IgG antibodies and the cytokine profile were determined by ELISA. Two weeks after the last immunization, all mice were orally challenged with 20 cysts of T. gondii Me49 strain and the number of brain cysts was determined. In addition, both humoral and cellular immune responses were also evaluated during the acute and chronic phase of T. gondii infection by ELISA.
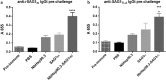
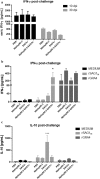
Results: The characterization of the immune response generated after vaccination with NbHsp90.3 as an adjuvant showed that NbHsp90.3-SAG1HC-immunized mice produced antibodies that were able to recognize not only rSAG1m but also the native SAG1 present in the total lysate antigen extract (SAG1TLA) from T. gondii tachyzoites, while control groups did not. Furthermore, anti-rSAG1m IgG2a/2b antibodies were significantly induced. In addition, only the spleen cell cultures from NbHsp90.3-SAG1HC-immunized mice showed a significantly increased production of IFN-γ. During the chronic phase of T. gondii infection, the antibodies generated by the infection were unable to detect the recombinant protein, but they did react with TLA extract. In addition, splenocytes from all groups showed a high production of IFN-γ when stimulated with rGRA4, but only those from NbHsp90.3-SAG1HC group stimulated with rSAG1m showed high production of IFN-γ. Finally, NbHsp90.3-SAG1HC-immunized mice exhibited a significant reduction in the cyst load (56%) against T. gondii infection.
Conclusions: We demonstrated that NbHsp90.3 enhances the humoral and cell-mediated immune response through a Th1 type cytokine production. Mice vaccinated with NbHsp90.3-SAG1HC exhibited a partial protection against T. gondii infection and it was correlated with the induction of memory immune response. We developed and validated a vaccine formulation which, to our knowledge, for the first time includes the NbHsp90.3 protein covalently fused to a peptide from T. gondii SAG1 protein that contains T- and B-cell epitopes.
Keywords: Adjuvant; Epitopes; Plant Hsp90; SAG1; Toxoplasma gondii; Toxoplasmosis; Vaccine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
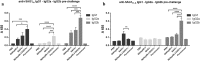

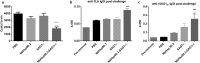
Similar articles
-
Immunization with plant-based vaccine expressing Toxoplasma gondii SAG1 fused to plant HSP90 elicits protective immune response in lambs.Acta Trop. 2025 Feb;262:107540. doi: 10.1016/j.actatropica.2025.107540. Epub 2025 Jan 31. Acta Trop. 2025. PMID: 39894243
-
Toxoplasma gondii: Vaccination with a DNA vaccine encoding T- and B-cell epitopes of SAG1, GRA2, GRA7 and ROP16 elicits protection against acute toxoplasmosis in mice.Vaccine. 2015 Nov 27;33(48):6757-62. doi: 10.1016/j.vaccine.2015.10.077. Epub 2015 Oct 27. Vaccine. 2015. PMID: 26518401
-
Oral immunization with a live recombinant attenuated Salmonella typhimurium protects mice against Toxoplasma gondii.Parasite Immunol. 2005 Jan-Feb;27(1-2):29-35. doi: 10.1111/j.1365-3024.2005.00738.x. Parasite Immunol. 2005. PMID: 15813720
-
Toxoplasma gondii surface antigen 1 (SAG1) as a potential candidate to develop vaccine against toxoplasmosis: A systematic review.Comp Immunol Microbiol Infect Dis. 2020 Apr;69:101414. doi: 10.1016/j.cimid.2020.101414. Epub 2020 Jan 7. Comp Immunol Microbiol Infect Dis. 2020. PMID: 31958746
-
A systematic review on efficiency of microneme proteins to induce protective immunity against Toxoplasma gondii.Eur J Clin Microbiol Infect Dis. 2019 Apr;38(4):617-629. doi: 10.1007/s10096-018-03442-6. Epub 2019 Jan 24. Eur J Clin Microbiol Infect Dis. 2019. PMID: 30680553
Cited by
-
Oral Immunization With a Plant HSP90-SAG1 Fusion Protein Produced in Tobacco Elicits Strong Immune Responses and Reduces Cyst Number and Clinical Signs of Toxoplasmosis in Mice.Front Plant Sci. 2021 Oct 4;12:726910. doi: 10.3389/fpls.2021.726910. eCollection 2021. Front Plant Sci. 2021. PMID: 34675949 Free PMC article.
-
Heat Shock Proteins 90 kDa: Immunomodulators and Adjuvants in Vaccine Design Against Infectious Diseases.Front Bioeng Biotechnol. 2021 Jan 20;8:622186. doi: 10.3389/fbioe.2020.622186. eCollection 2020. Front Bioeng Biotechnol. 2021. PMID: 33553125 Free PMC article. Review.
-
Advances in Toxoplasma gondii Vaccines: Current Strategies and Challenges for Vaccine Development.Vaccines (Basel). 2021 Apr 21;9(5):413. doi: 10.3390/vaccines9050413. Vaccines (Basel). 2021. PMID: 33919060 Free PMC article. Review.
-
Design and immunological evaluation of a multi-epitope vaccine candidate against Toxoplasma gondii incorporating MIC13, GRA1, and SAG1 antigens in BALB/c mice.Food Waterborne Parasitol. 2025 Jun 2;40:e00269. doi: 10.1016/j.fawpar.2025.e00269. eCollection 2025 Sep. Food Waterborne Parasitol. 2025. PMID: 40546389 Free PMC article.
-
Cyclic di AMP phosphodiesterase nanovaccine elicits protective immunity against Burkholderia cenocepacia infection in mice.NPJ Vaccines. 2025 Feb 1;10(1):22. doi: 10.1038/s41541-025-01074-4. NPJ Vaccines. 2025. PMID: 39893156 Free PMC article.
References
-
- Kasper LH, Crabb JH, Pfefferkorn ER. Purification of a major membrane protein of Toxoplasma gondii by immunoabsorption with a monoclonal antibody. J Immunol. 1983;130:2407–2412. - PubMed
-
- Clemente M. Plant-based vaccines against toxoplasmosis. In: Rosales-Mendoza S, editor. Genetically engineered plants as a source of vaccines against wide spread diseases. New York: Springer International Publishing; 2014. pp. 214–241.
-
- Sander VA, Angel SO, Clemente M. A comprehensive review of Toxoplasma gondii biology and host-cell interaction: challenges for a plant-based vaccine. In: MacDonald J, editor. Prospects of plant-based vaccines in veterinary medicine. Basel: Springer International Publishing; 2018. pp. 89–120.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

