Three-dimensional visualisation of the fetal heart using prenatal MRI with motion-corrected slice-volume registration: a prospective, single-centre cohort study
- PMID: 30910324
- PMCID: PMC6484696
- DOI: 10.1016/S0140-6736(18)32490-5
Three-dimensional visualisation of the fetal heart using prenatal MRI with motion-corrected slice-volume registration: a prospective, single-centre cohort study
Erratum in
-
Department of Error.Lancet. 2022 Apr 23;399(10335):1606. doi: 10.1016/S0140-6736(22)00702-4. Lancet. 2022. PMID: 35461557 Free PMC article. No abstract available.
-
Department of Error.Lancet. 2022 May 21;399(10339):1940. doi: 10.1016/S0140-6736(22)00913-8. Lancet. 2022. PMID: 35598623 Free PMC article. No abstract available.
Abstract
Background: Two-dimensional (2D) ultrasound echocardiography is the primary technique used to diagnose congenital heart disease before birth. There is, however, a longstanding need for a reliable form of secondary imaging, particularly in cases when more detailed three-dimensional (3D) vascular imaging is required, or when ultrasound windows are of poor diagnostic quality. Fetal MRI, which is well established for other organ systems, is highly susceptible to fetal movement, particularly for 3D imaging. The objective of this study was to investigate the combination of prenatal MRI with novel, motion-corrected 3D image registration software, as an adjunct to fetal echocardiography in the diagnosis of congenital heart disease.
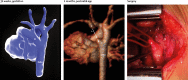
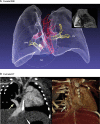
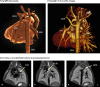
Methods: Pregnant women carrying a fetus with known or suspected congenital heart disease were recruited via a tertiary fetal cardiology unit. After initial validation experiments to assess the general reliability of the approach, MRI data were acquired in 85 consecutive fetuses, as overlapping stacks of 2D images. These images were then processed with a bespoke open-source reconstruction algorithm to produce a super-resolution 3D volume of the fetal thorax. These datasets were assessed with measurement comparison with paired 2D ultrasound, structured anatomical assessment of the 2D and 3D data, and contemporaneous, archived clinical fetal MRI reports, which were compared with postnatal findings after delivery.
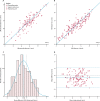
Findings: Between Oct 8, 2015, and June 30, 2017, 101 patients were referred for MRI, of whom 85 were eligible and had fetal MRI. The mean gestational age at the time of MRI was 32 weeks (range 24-36). High-resolution (0·50-0·75 mm isotropic) 3D datasets of the fetal thorax were generated in all 85 cases. Vascular measurements showed good overall agreement with 2D echocardiography in 51 cases with paired data (intra-class correlation coefficient 0·78, 95% CI 0·68-0·84), with fetal vascular structures more effectively visualised with 3D MRI than with uncorrected 2D MRI (657 [97%] of 680 anatomical areas identified vs 358 [53%] of 680 areas; p<0·0001). When a structure of interest was visualised in both 2D and 3D data (n=358), observers gave a higher diagnostic quality score for 3D data in 321 (90%) of cases, with 37 (10%) scores tied with 2D data, and no lower scores than for 2D data (Wilcoxon signed rank test p<0·0001). Additional anatomical features were described in ten cases, of which all were confirmed postnatally.
Interpretation: Standard fetal MRI with open-source image processing software is a reliable method of generating high-resolution 3D imaging of the fetal vasculature. The 3D volumes produced show good spatial agreement with ultrasound, and significantly improved visualisation and diagnostic quality compared with source 2D MRI data. This freely available combination requires minimal infrastructure, and provides safe, powerful, and highly complementary imaging of the fetal cardiovascular system.
Funding: Wellcome Trust/EPSRC Centre for Medical Engineering, National Institute for Health Research.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Fetal MRI and prenatal diagnosis of congenital heart defects.Lancet. 2019 Apr 20;393(10181):1574-1576. doi: 10.1016/S0140-6736(18)32853-8. Epub 2019 Mar 22. Lancet. 2019. PMID: 30910326 No abstract available.
-
Improved visualization of fetal heart abnormalities with 3D MRI.Nat Rev Cardiol. 2019 Jul;16(7):386. doi: 10.1038/s41569-019-0199-9. Nat Rev Cardiol. 2019. PMID: 30948785 No abstract available.
References
-
- Hunter LE, Simpson JM. Prenatal screening for structural congenital heart disease. Nat Rev Cardiol. 2014;11:323–334. - PubMed
-
- Bensemlali M, Stirnemann J, Le Bidois J. Discordances Between Pre-Natal and Post-Natal Diagnoses of Congenital Heart Diseases and Impact on Care Strategies. J Am Coll Cardiol. 2016;68:921–930. - PubMed
-
- Heymann MA, Rudolph AM. Effects of congenital heart disease on fetal and neonatal circulations. Prog Cardiovasc Dis. 1972;15:115–143. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

