Phosphatidate-mediated regulation of lipid synthesis at the nuclear/endoplasmic reticulum membrane
- PMID: 30910690
- PMCID: PMC6755077
- DOI: 10.1016/j.bbalip.2019.03.006
Phosphatidate-mediated regulation of lipid synthesis at the nuclear/endoplasmic reticulum membrane
Abstract
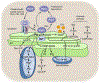
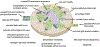
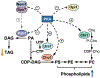
In yeast and higher eukaryotes, phospholipids and triacylglycerol are derived from phosphatidate at the nuclear/endoplasmic reticulum membrane. In de novo biosynthetic pathways, phosphatidate is channeled into membrane phospholipids via its conversion to CDP-diacylglycerol. Its dephosphorylation to diacylglycerol is required for the synthesis of triacylglycerol as well as for the synthesis of phosphatidylcholine and phosphatidylethanolamine via the Kennedy pathway. In addition to the role of phosphatidate as a precursor, it is a regulatory molecule in the transcriptional control of phospholipid synthesis genes via the Henry regulatory circuit. Pah1 phosphatidate phosphatase and Dgk1 diacylglycerol kinase are key players that function counteractively in the control of the phosphatidate level at the nuclear/endoplasmic reticulum membrane. Loss of Pah1 phosphatidate phosphatase activity not only affects triacylglycerol synthesis but also disturbs the balance of the phosphatidate level, resulting in the alteration of lipid synthesis and related cellular defects. The pah1Δ phenotypes requiring Dgk1 diacylglycerol kinase exemplify the importance of the phosphatidate level in the misregulation of cellular processes. The catalytic function of Pah1 requires its translocation from the cytoplasm to the nuclear/endoplasmic reticulum membrane, which is regulated through its phosphorylation in the cytoplasm by multiple protein kinases as well as through its dephosphorylation by the membrane-associated Nem1-Spo7 protein phosphatase complex. This article is part of a Special Issue entitled Endoplasmic reticulum platforms for lipid dynamics edited by Shamshad Cockcroft and Christopher Stefan.
Keywords: Diacylglycerol; Diacylglycerol kinase; Phosphatidate; Phosphatidate phosphatase; Phospholipid; Triacylglycerol.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures
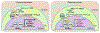
References
-
- Vance DE. Glycerolipid biosynthesis in eukaryotes In Biochemistry of Lipids, Lipoproteins and Membranes, (Vance DE and Vance J, eds) 5th Ed, Elsevier Science Publishers B.V., Amsterdam: (2004) pp.153–181.
-
- Hakomori S, Igarashi Y, Functional role of glycosphingolipids in cell recognition and signaling, J. Biochem 118 (1995) 1091–1103. - PubMed
-
- van MG, de Kroon AI, Lipid map of the mammalian cell, J. Cell Sci 124 (2011) 5–8. - PubMed
-
- Pomorski T, Hrafnsdottir S, Devaux PF, van MG, Lipid distribution and transport across cellular membranes, Semin. Cell Dev. Biol 12 (2001) 139–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

