Location-dependent maintenance of intrinsic susceptibility to mTORC1-driven tumorigenesis
- PMID: 30910807
- PMCID: PMC6435042
- DOI: 10.26508/lsa.201800218
Location-dependent maintenance of intrinsic susceptibility to mTORC1-driven tumorigenesis
Abstract
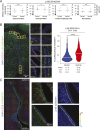
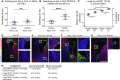
Neural stem/progenitor cells (NSPCs) of the ventricular-subventricular zone (V-SVZ) are candidate cells of origin for many brain tumors. However, whether NSPCs in different locations within the V-SVZ differ in susceptibility to tumorigenic mutations is unknown. Here, single-cell measurements of signal transduction intermediates in the mechanistic target of rapamycin complex 1 (mTORC1) pathway reveal that ventral NSPCs have higher levels of signaling than dorsal NSPCs. These features are linked with differences in mTORC1-driven disease severity: introduction of a pathognomonic Tsc2 mutation only results in formation of tumor-like masses from the ventral V-SVZ. We propose a direct link between location-dependent intrinsic growth properties imbued by mTORC1 and predisposition to tumor development.
© 2019 Rushing et al.
Conflict of interest statement
JM Irish is a co-founder and board member at Cytobank Inc. and received research support from Incyte Corp., Janssen, and Pharmacyclics. The other authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- P60 DK020593/DK/NIDDK NIH HHS/United States
- S10 OD023475/OD/NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- F31 NS096908/NS/NINDS NIH HHS/United States
- R01 NS078289/NS/NINDS NIH HHS/United States
- R01 NS096238/NS/NINDS NIH HHS/United States
- R00 CA143231/CA/NCI NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- S10 OD021630/OD/NIH HHS/United States
- U2C DK059637/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- T32 CA009592/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
