Evidence for a regulated Ca2+ entry in proximal tubular cells and its implication in calcium stone formation
- PMID: 30910829
- PMCID: PMC6526711
- DOI: 10.1242/jcs.225268
Evidence for a regulated Ca2+ entry in proximal tubular cells and its implication in calcium stone formation
Abstract
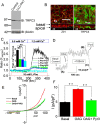
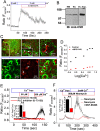
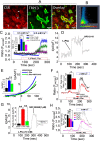
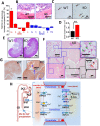
Calcium phosphate (CaP) crystals, which begin to form in the early segments of the loop of Henle (LOH), are known to act as precursors for calcium stone formation. The proximal tubule (PT), which is just upstream of the LOH and is a major site for Ca2+ reabsorption, could be a regulator of such CaP crystal formation. However, PT Ca2+ reabsorption is mostly described as being paracellular. Here, we show the existence of a regulated transcellular Ca2+ entry pathway in luminal membrane PT cells induced by Ca2+-sensing receptor (CSR, also known as CASR)-mediated activation of transient receptor potential canonical 3 (TRPC3) channels. In support of this idea, we found that both CSR and TRPC3 are physically and functionally coupled at the luminal membrane of PT cells. More importantly, TRPC3-deficient mice presented with a deficiency in PT Ca2+ entry/transport, elevated urinary [Ca2+], microcalcifications in LOH and urine microcrystals formations. Taken together, these data suggest that a signaling complex comprising CSR and TRPC3 exists in the PT and can mediate transcellular Ca2+ transport, which could be critical in maintaining the PT luminal [Ca2+] to mitigate formation of the CaP crystals in LOH and subsequent formation of calcium stones.
Keywords: Ca2+ channel; Ca2+ signaling; Calcium phosphate stone; Loop of Henle; Renal calcium transport.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
-
- Bandyopadhyay B. C., Ong H. L., Lockwich T. P., Liu X., Paria B. C., Singh B. B. and Ambudkar I. S. (2008). TRPC3 controls agonist-stimulated intracellular Ca2+ release by mediating the interaction between inositol 1, 4, 5-trisphosphate receptor and RACK1. J. Biol. Chem. 283, 32821-32830. 10.1074/jbc.M805382200 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

