Muscle-derived miR-34a increases with age in circulating extracellular vesicles and induces senescence of bone marrow stem cells
- PMID: 30910993
- PMCID: PMC6461183
- DOI: 10.18632/aging.101874
Muscle-derived miR-34a increases with age in circulating extracellular vesicles and induces senescence of bone marrow stem cells
Abstract
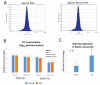
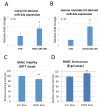
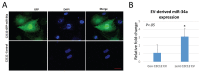
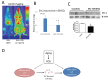
Extracellular vesicles (EVs) are known to play important roles in cell-cell communication. Here we investigated the role of muscle-derived EVs and their microRNAs in the loss of bone stem cell populations with age. Aging in male and female C57BL6 mice was associated with a significant increase in expression of the senescence-associated microRNA miR-34a-5p (miR-34a) in skeletal muscle and in serum -derived EVs. Muscle-derived, alpha-sarcoglycan positive, EVs isolated from serum samples also showed a significant increase in miR-34a with age. EVs were isolated from conditioned medium of C2C12 mouse myoblasts and primary human myotubes after cells were treated with hydrogen peroxide to simulate oxidative stress. These EVs were shown to have elevated levels of miR-34a, and these EVs decreased viability of bone marrow mesenchymal (stromal) cells (BMSCs) and increased BMSC senescence. A lentiviral vector system was used to overexpress miR-34a in C2C12 cells, and EVs isolated from these transfected cells were observed to home to bone in vivo and to induce senescence and decrease Sirt1 expression of primary bone marrow cells ex vivo. These findings suggest that aged skeletal muscle is a potential source of circulating, senescence-associated EVs that may directly impact stem cell populations in tissues such as bone via their microRNA cargo.
Keywords: exosomes; muscle-bone crosstalk; osteoporosis; sarcopenia; senescence.
Conflict of interest statement
Figures



References
-
- Hubal MJ, Nadler EP, Ferrante SC, Barberio MD, Suh JH, Wang J, Dohm GL, Pories WJ, Mietus-Snyder M, Freishtat RJ. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring). 2017; 25:102–10. 10.1002/oby.21709 - DOI - PMC - PubMed
-
- Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, et al.. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018; 27:237–251.e4. 10.1016/j.cmet.2017.12.001 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

