Choline kinase inhibitors EB-3D and EB-3P interferes with lipid homeostasis in HepG2 cells
- PMID: 30911014
- PMCID: PMC6433853
- DOI: 10.1038/s41598-019-40885-z
Choline kinase inhibitors EB-3D and EB-3P interferes with lipid homeostasis in HepG2 cells
Abstract
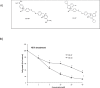
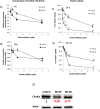
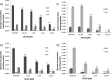
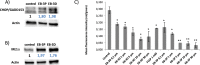
A full understanding of the molecular mechanism of action of choline kinase α (ChoKα) inhibitors at the cell level is essential for developing therapeutic and preventive approaches for cancer. The aim of the present study was to evaluate the effects of the ChoKα inhibitors EB-3D and EB-3P on lipid metabolism in HepG2 cells. We used [methyl-14C]choline, [1,2-14C]acetic acid and [2-3H]glycerol as exogenous precursors of the corresponding phospholipids and neutral lipids. [Methyl-14C]choline was also used to determine choline uptake. Protein levels were determined by Western blot. Ultrastructural alterations were investigated by transmission electron microscopy. In this work, we demonstrate that EB-3D and EB-3P interfere with phosphatidylcholine biosynthesis via both CDP-choline pathway and choline uptake by the cell. Moreover, the synthesis of both diacylglycerols and triacylglycerols was affected by cell exposure to both inhibitors. These effects were accompanied by a substantial decrease in cholesterol biosynthesis, as well as alterations in the expression of proteins related to cholesterol homeostasis. We also found that EB-3D and EB-3P lowered ChoKα protein levels. All these effects could be explained by the modulation of the AMP-activated protein kinase signalling pathway. We show that both inhibitors cause mitochondrial alteration and an endoplasmic reticulum stress response. EB-3D and EB-3P exert effects on ChoKα expression, AMPK activation, apoptosis, endoplasmic reticulum stress and lipid metabolism. Taken together, results show that EB-3D and EB-3P have potential anti-cancer activity through the deregulation of lipid metabolism.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

