Soy isoflavones and their metabolites modulate cytokine-induced natural killer cell function
- PMID: 30911044
- PMCID: PMC6433892
- DOI: 10.1038/s41598-019-41687-z
Soy isoflavones and their metabolites modulate cytokine-induced natural killer cell function
Abstract
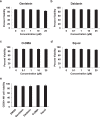
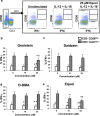
Soybeans are a rich source of isoflavones that have been linked with anti-inflammatory processes and various health benefits. However, specific mechanisms whereby soy bioactives impact immune cell subsets are unclear. Isoflavones, such as genistein and daidzein, are metabolized by microbes to bioactive metabolites as O-desmethylangolensin (O-DMA) and equol, whose presence has been linked to health benefits. We examined how soy isoflavones and metabolites impact natural killer (NK) cell signaling and function. We observe no impact of isoflavones on viability of healthy donor peripheral blood mononuclear cells (PBMCs) or NK cells, even at high (25 µM) concentrations. However, pre-treatment of PBMCs with physiologically-relevant concentrations of genistein (p = 0.0023) and equol (p = 0.006) decreases interleukin (IL)-12/IL-18-induced interferon-gamma (IFN-γ) production versus controls. Detailed cellular analyses indicate genistein and equol decrease IL-12/IL-18-induced IFN-γ production by human NK cell subsets, but do not consistently alter cytotoxicity. At the level of signal transduction, genistein decreases IL-12/IL-18-induced total phosphorylated tyrosine, and phosphorylation MAPK pathway components. Further, genistein limits IL-12/IL-18-mediated upregulation of IL-18Rα expression on NK cells (p = 0.0109). Finally, in vivo studies revealed that C57BL/6 mice fed a soy-enriched diet produce less plasma IFN-γ following administration of IL-12/IL-18 versus control-fed animals (p < 0.0001). This study provides insight into how dietary soy modulates NK cell functions.
Conflict of interest statement
Dr. Lesinski has consulted for ProDa Biotech, LLC and received compensation. Dr. Lesinski has received research funding through a sponsored research agreements between Emory University and Merck and Co., Inc., Boehringer Ingelheim, Inc., Bristol Myers Squibb, Inc. and Vaccinex, Inc. that are not related to this work. All other authors declare no conflicts of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

