Immunological Agents Used in Cancer Treatment
- PMID: 30911265
- PMCID: PMC6422631
- DOI: 10.5152/eurasianjmed.2018.18194
Immunological Agents Used in Cancer Treatment
Abstract
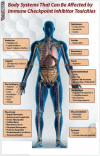
Immune checkpoint inhibitors (ICI) are monoclonal antibodies targeting cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed cell death protein-1 (PD-1), or PD-1 ligand (PD-L1). ICI are approved for the treatment of malign melanoma, non-small cell lung cancer, classical Hodgkin lymphoma, head and neck squamous cell carcinoma, urothelial carcinoma, and renal cell carcinoma. They can lead to long-term anti-tumor responses by deactivating the brake mechanism in the immune system. Ipilimumab, tremelimumab, pembrolizumab, nivolumab, atezolizumab, durvalumab, and avelumab are examples of ICI. CTLA-4 is a brake mechanism in immune response. Ipilimumab and tremelimumab are antibodies against CTLA-4. PD-1 is another important immune checkpoint co-inhibitor receptor that is expressed by activated T cells in the peripheral tissue. As a result of blockage of the PD-1/PD-L1 pathway, local tumor-specific immune response augments, and long-term tumor control can be achieved. In recent years, ICI are approved for the treatment of various malignities. They may be responsible for specific toxicities called immune-related adverse events (irAEs). irAEs are a consequence infiltration of normal tissues by activated T lymphocytes that are responsible for autoimmunity. Corticosteroids and anti-tumor necrosis factor agents, such as infliximab and mycophenolate mofetil, are effective in the treatment of irAEs. Immune checkpoint inhibition with monoclonal antibodies against CTLA-4 and/or PD-1/PD-L1 by single agent or combination treatments became a new option in various solid tumors. However, ICI have unique adverse events, and these adverse events should be considered in any new onset clinical situation and should be managed properly. Future prospective randomized clinical trials will clarify recent questions.
Keywords: Immunotherapy; cancer; checkpoint inhibition.
Conflict of interest statement
Conflict of Interest: The authors have no conflicts of interest to declare.
Figures
References
-
- European Medicines Agency. EMEA/H/C/002213-PSUSA/00009200/201409 - ipilimumab product information. Aug, 2015. Available From: URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info....
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials