Radiation: a poly-traumatic hit leading to multi-organ injury
- PMID: 30911370
- PMCID: PMC6417034
- DOI: 10.1186/s13578-019-0286-y
Radiation: a poly-traumatic hit leading to multi-organ injury
Abstract
The range of radiation threats we face today includes everything from individual radiation exposures to mass casualties resulting from a terrorist incident, and many of these exposure scenarios include the likelihood of additional traumatic injury as well. Radiation injury is defined as an ionizing radiation exposure inducing a series of organ injury within a specified time. Severity of organ injury depends on the radiation dose and the duration of radiation exposure. Organs and cells with high sensitivity to radiation injury are the skin, the hematopoietic system, the gastrointestinal (GI) tract, spermatogenic cells, and the vascular system. In general, acute radiation syndrome (ARS) includes DNA double strand breaks (DSB), hematopoietic syndrome (bone marrow cells and circulatory cells depletion), cutaneous injury, GI death, brain hemorrhage, and splenomegaly within 30 days after radiation exposure. Radiation injury sensitizes target organs and cells resulting in ARS. Among its many effects on tissue integrity at various levels, radiation exposure results in activation of the iNOS/NF-kB/NF-IL6 and p53/Bax pathways; and increases DNA single and double strand breaks, TLR signaling, cytokine concentrations, bacterial infection, cytochrome c release from mitochondria to cytoplasm, and possible PARP-dependent NAD and ATP-pool depletion. These alterations lead to apoptosis and autophagy and, as a result, increased mortality. In this review, we summarize what is known about how radiation exposure leads to the radiation response with time. We also describe current and prospective countermeasures relevant to the treatment and prevention of radiation injury.
Keywords: AKT; Acute GI death; Acute hematopoietic syndrome; Apoptosis; Autophagy; Brain injury; DNA damage; Free radical; Hemorrhage; MAPK; NF-IL6; NF-kB; PARP; Radiation injury; STAT3; iNOS.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
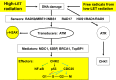

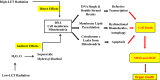

References
-
- Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177(3):229–243. - PubMed
-
- Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6(9):702–713. - PubMed
-
- Hall EJ, Giacca AJ. Radiobiology for the radiologist. 6. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 252–268.
-
- Fliedner TM, Graessel D, Meineke V, Dorr H. Pathological principles underlying the blood cell concentration responses used to assess the severity of effect after accidental whole-body radiation exposure: an essential basis for an evidence-based clinical triage. Exp Hematol. 2007;35(4 Suppl 1):8–16. - PubMed
-
- MacNaughton WK. Review article: new insights into the pathologenesis of radiation-induced intestinal dysfunction. Aliment Pharmacol Ther. 2000;14(5):523–528. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

