Anti-PrPC antibody rescues cognition and synapses in transgenic alzheimer mice
- PMID: 30911579
- PMCID: PMC6414488
- DOI: 10.1002/acn3.730
Anti-PrPC antibody rescues cognition and synapses in transgenic alzheimer mice
Abstract
Objective: Amyloid-beta oligomers (Aßo) trigger the development of Alzheimer's disease (AD) pathophysiology. Cellular prion protein (PrPC) initiates synaptic damage as a high affinity receptor for Aßo. Here, we evaluated the preclinical therapeutic efficacy of a fully human monoclonal antibody against PrPC. This AZ59 antibody selectively targets the Aβo binding site in the amino-terminal unstructured domain of PrPC to avoid any potential risk of direct toxicity.
Methods: Potency of AZ59 was evaluated by binding to PrPC, blockade of Aβo interaction and interruption of Aβo signaling. AZ59 was administered to mice by weekly intraperitoneal dosing and brain antibody measured. APP/PS1 transgenic mice were treated with AZ59 and assessed by memory tests, by brain biochemistry and by histochemistry for Aß, gliosis and synaptic density.
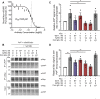
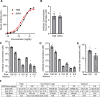
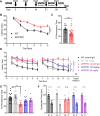
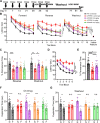
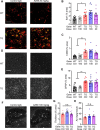
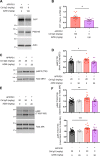
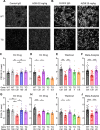
Results: AZ59 binds PrPC with 100 pmol/L affinity and blocks human brain Aßo binding to PrPC, as well as prevents synaptotoxic signaling. Weekly i.p. dosing of 20 mg/kg AZ59 in a murine form achieves trough brain antibody levels greater than 10 nmol/L. Aged symptomatic APP/PS1 transgenic mice treated with AZ59 for 5-7 weeks show a full rescue of behavioral and synaptic loss phenotypes. This recovery occurs without clearance of plaque pathology or elimination of gliosis. AZ59 treatment also normalizes synaptic signaling abnormalities in transgenic brain. These benefits are dose-dependent and persist for at least 1 month after the last dose.
Interpretation: Preclinical data demonstrate that systemic AZ59 therapy rescues central synapses and memory function from transgenic Alzheimer's disease pathology, supporting a disease-modifying therapeutic potential.
Conflict of interest statement
J.H., G.R., B.D., M.G., M.G., C.D., T.V., I.C., and A.B. are full time employees of AstraZeneca MedImmune and have filed a patent application describing AZ59 antibody. S.M.S. is Inventor for an issued patent claiming use of PrPC inhibition for Alzheimer's disease.
Figures

References
-
- Citron M, Westaway D, Xia W, et al. Mutant presenilins of Alzheimer's disease increase production of 42‐residue amyloid beta‐protein in both transfected cells and transgenic mice. Nat Med 1997;3:67–72. - PubMed
-
- Cleary JP, Walsh DM, Hofmeister JJ, et al. Natural oligomers of the amyloid‐beta protein specifically disrupt cognitive function. Nat Neurosci 2005;8:79–84. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002;297:353–356. - PubMed
-
- Giacobini E, Gold G. Alzheimer disease therapy–moving from amyloid‐beta to tau. Nat Rev Neurol 2013;9:677–686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

