Diagnostic and therapeutic approach to infectious diseases in solid organ transplant recipients
- PMID: 30911807
- PMCID: PMC7079836
- DOI: 10.1007/s00134-019-05597-y
Diagnostic and therapeutic approach to infectious diseases in solid organ transplant recipients
Abstract
Purpose: Prognosis of solid organ transplant (SOT) recipients has improved, mainly because of better prevention of rejection by immunosuppressive therapies. However, SOT recipients are highly susceptible to conventional and opportunistic infections, which represent a major cause of morbidity, graft dysfunction and mortality.
Methods: Narrative review.
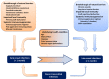
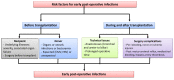
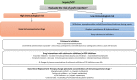
Results: We cover the current epidemiology and main aspects of infections in SOT recipients including risk factors such as postoperative risks and specific risks for different transplant recipients, key points on anti-infective prophylaxis as well as diagnostic and therapeutic approaches. We provide an up-to-date guide for management of the main syndromes that can be encountered in SOT recipients including acute respiratory failure, sepsis or septic shock, and central nervous system infections as well as bacterial infections with multidrug-resistant strains, invasive fungal diseases, viral infections and less common pathogens that may impact this patient population.
Conclusion: We provide state-of the art review of available knowledge of critically ill SOT patients with infections.
Keywords: Immunocompromized; Outcome; Sepsis; Septic shock; Solid organ recipient.
Conflict of interest statement
MB has participated in advisory boards and/or received speaker honoraria from Achaogen, Angelini, Astellas, AstraZeneca, Bayer, Basilea, Biomerieux, Cidara, Gilead, Menarini, MSD, Nabriva, Paratek, Pfizer, Roche, The Medicine Company, Shionogi, Tetraphase, VenatoRx and Vifor. RF reports participation in scientific advisory boards: MSD, Shionogi and lectures: Beckton-Dickinson, MSD, Astelas, Pfizer, Thermo, Estor. SJ reports receiving consulting fees from Drager, Hamilton, Maquet, Medtronic and Fisher & Paykel. CEL reports participations in advisory boards (Bayer Healthcare, Carmat, Faron, ThermoFischer Brahms) and lectures (MSD, Nihon-Koden, Biomérieux). MM has received payment for lectures, advisory board participation and travel expenses from MSD, Jansen, Pfizer, Astelas, Gilead, all outside the submitted work. FP reports lecture fees paid to his institution by ALEXION. JFT reports participation to scientific advisory boards (Astra-Zeneca, Pfizer, MSD, Nabriva, Gilead), lectures (Biomerieux, MSD, Astelas, 3M, Pfizer) and scientific grants (MSD, Pfizer). CV reports personal fees from MSD Int, Gilead, Pfizer, Angelini, Astelas and Basilea. LZ reports scientific grants not related to the review by Jazz Pharmaceuticals.
Figures

References
-
- Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, Meiser B, Rossano JW, Chambers DC, Yusen RD, Stehlik J. The registry of the international society for heart and lung transplantation: thirty-fourth adult heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2017;36:1037–1046. doi: 10.1016/j.healun.2017.07.019. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

