Causes, consequences, and therapy of tumors acidosis
- PMID: 30911978
- PMCID: PMC6625890
- DOI: 10.1007/s10555-019-09792-7
Causes, consequences, and therapy of tumors acidosis
Abstract
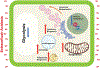
While cancer is commonly described as "a disease of the genes," it is also associated with massive metabolic reprogramming that is now accepted as a disease "Hallmark." This programming is complex and often involves metabolic cooperativity between cancer cells and their surrounding stroma. Indeed, there is emerging clinical evidence that interrupting a cancer's metabolic program can improve patients' outcomes. The most commonly observed and well-studied metabolic adaptation in cancers is the fermentation of glucose to lactic acid, even in the presence of oxygen, also known as "aerobic glycolysis" or the "Warburg Effect." Much has been written about the mechanisms of the Warburg effect, and this remains a topic of great debate. However, herein, we will focus on an important sequela of this metabolic program: the acidification of the tumor microenvironment. Rather than being an epiphenomenon, it is now appreciated that this acidosis is a key player in cancer somatic evolution and progression to malignancy. Adaptation to acidosis induces and selects for malignant behaviors, such as increased invasion and metastasis, chemoresistance, and inhibition of immune surveillance. However, the metabolic reprogramming that occurs during adaptation to acidosis also introduces therapeutic vulnerabilities. Thus, tumor acidosis is a relevant therapeutic target, and we describe herein four approaches to accomplish this: (1) neutralizing acid directly with buffers, (2) targeting metabolic vulnerabilities revealed by acidosis, (3) developing acid-activatable drugs and nanomedicines, and (4) inhibiting metabolic processes responsible for generating acids in the first place.
Keywords: Anti-acidic therapy; Cancer; Exosomes; Microenvironment acidity.
Figures
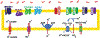


References
-
- Toyomura T, Oka T, Yamaguchi C, Wada Y & Futai M Three subunit a isoforms of mouse vacuolar H(+)-ATPase. Preferential expression of the a3 isoform during osteoclast differentiation. J Biol Chem 275, 8760–5 (2000). - PubMed
-
- Boedtkjer E et al. Contribution of Na+,HCO3−-cotransport to cellular pH control in human breast cancer: a role for the breast cancer susceptibility locus NBCn1 (SLC4A7). Int J Cancer 132, 1288–1299 (2013). - PubMed
-
- Lee S et al. Na+,HCO3−-cotransport is functionally upregulated during human breast carcinogenesis and required for the inverted pH gradient across the plasma membrane. Pflugers Arch 467, 367–377 (2015). - PubMed
-
- Lee S et al. Disrupting Na+,HCO3−-cotransporter NBCn1 (Slc4a7) delays murine breast cancer development. Oncogene 35, 2112–22 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

