Vaccines for preventing rotavirus diarrhoea: vaccines in use
- PMID: 30912133
- PMCID: PMC6434239
- DOI: 10.1002/14651858.CD008521.pub4
Vaccines for preventing rotavirus diarrhoea: vaccines in use
Update in
-
Vaccines for preventing rotavirus diarrhoea: vaccines in use.Cochrane Database Syst Rev. 2019 Oct 28;2019(10):CD008521. doi: 10.1002/14651858.CD008521.pub5. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Nov 17;11:CD008521. doi: 10.1002/14651858.CD008521.pub6. PMID: 31684685 Free PMC article. Updated.
Abstract
Background: Rotavirus results in more diarrhoea-related deaths in children under five years than any other single agent in countries with high childhood mortality. It is also a common cause of diarrhoea-related hospital admissions in countries with low childhood mortality. Rotavirus vaccines that have been prequalified by the World Health Organization (WHO) include a monovalent vaccine (RV1; Rotarix, GlaxoSmithKline), a pentavalent vaccine (RV5; RotaTeq, Merck), and, more recently, another monovalent vaccine (Rotavac, Bharat Biotech).
Objectives: To evaluate rotavirus vaccines prequalified by the WHO (RV1, RV5, and Rotavac) for their efficacy and safety in children.
Search methods: On 4 April 2018 we searched MEDLINE (via PubMed), the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (published in the Cochrane Library), Embase, LILACS, and BIOSIS. We also searched the WHO ICTRP, ClinicalTrials.gov, clinical trial reports from manufacturers' websites, and reference lists of included studies and relevant systematic reviews.
Selection criteria: We selected randomized controlled trials (RCTs) in children comparing rotavirus vaccines prequalified for use by the WHO versus placebo or no intervention.
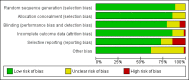
Data collection and analysis: Two review authors independently assessed trial eligibility and assessed risks of bias. One review author extracted data and a second author cross-checked them. We combined dichotomous data using the risk ratio (RR) and 95% confidence interval (CI). We stratified the analysis by country mortality rate and used GRADE to evaluate evidence certainty.
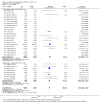
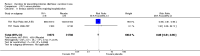
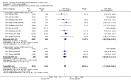
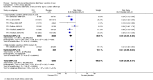
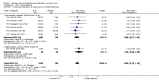
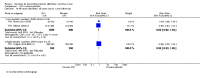
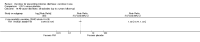
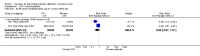
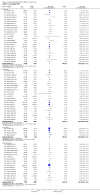
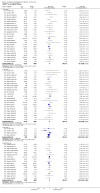
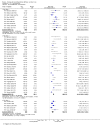
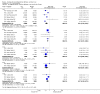
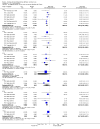
Main results: Fifty-five trials met the inclusion criteria and enrolled a total of 216,480 participants. Thirty-six trials (119,114 participants) assessed RV1, 15 trials (88,934 participants) RV5, and four trials (8432 participants) Rotavac.RV1 Children vaccinated and followed up the first year of life In low-mortality countries, RV1 prevents 84% of severe rotavirus diarrhoea cases (RR 0.16, 95% CI 0.09 to 0.26; 43,779 participants, 7 trials; high-certainty evidence), and probably prevents 41% of cases of severe all-cause diarrhoea (RR 0.59, 95% CI 0.47 to 0.74; 28,051 participants, 3 trials; moderate-certainty evidence). In high-mortality countries, RV1 prevents 63% of severe rotavirus diarrhoea cases (RR 0.37, 95% CI 0.23 to 0.60; 6114 participants, 3 trials; high-certainty evidence), and 27% of severe all-cause diarrhoea cases (RR 0.73, 95% CI 0.56 to 0.95; 5639 participants, 2 trials; high-certainty evidence).Children vaccinated and followed up for two yearsIn low-mortality countries, RV1 prevents 82% of severe rotavirus diarrhoea cases (RR 0.18, 95% CI 0.14 to 0.23; 36,002 participants, 9 trials; high-certainty evidence), and probably prevents 37% of severe all-cause diarrhoea episodes (rate ratio 0.63, 95% CI 0.56 to 0.71; 39,091 participants, 2 trials; moderate-certainty evidence). In high-mortality countries RV1 probably prevents 35% of severe rotavirus diarrhoea cases (RR 0.65, 95% CI 0.51 to 0.83; 13,768 participants, 2 trials; high-certainty evidence), and 17% of severe all-cause diarrhoea cases (RR 0.83, 95% CI 0.72 to 0.96; 2764 participants, 1 trial; moderate-certainty evidence).No increased risk of serious adverse events (SAE) was detected (RR 0.88 95% CI 0.83 to 0.93; high-certainty evidence). There were 30 cases of intussusception reported in 53,032 children after RV1 vaccination and 28 cases in 44,214 children after placebo or no intervention (RR 0.70, 95% CI 0.46 to 1.05; low-certainty evidence).RV5 Children vaccinated and followed up the first year of life In low-mortality countries, RV5 probably prevents 92% of severe rotavirus diarrhoea cases (RR 0.08, 95% CI 0.03 to 0.22; 4132 participants, 5 trials; moderate-certainty evidence). We did not identify studies reporting on severe all-cause diarrhoea in low-mortality countries. In high-mortality countries, RV5 prevents 57% of severe rotavirus diarrhoea (RR 0.43, 95% CI 0.29 to 0.62; 5916 participants, 2 trials; high-certainty evidence), but there is probably little or no difference between vaccine and placebo for severe all-cause diarrhoea (RR 0.80, 95% CI 0.58 to 1.11; 1 trial, 4085 participants; moderate-certainty evidence).Children vaccinated and followed up for two yearsIn low-mortality countries, RV5 prevents 82% of severe rotavirus diarrhoea cases (RR 0.18, 95% CI 0.08 to 0.39; 7318 participants, 4 trials; moderate-certainty evidence). We did not identify studies reporting on severe all-cause diarrhoea in low-mortality countries. In high-mortality countries, RV5 prevents 41% of severe rotavirus diarrhoea cases (RR 0.59, 95% CI 0.43 to 0.82; 5885 participants, 2 trials; high-certainty evidence), and 15% of severe all-cause diarrhoea cases (RR 0.85, 95% CI 0.75 to 0.98; 5977 participants, 2 trials; high-certainty evidence).No increased risk of serious adverse events (SAE) was detected (RR 0.93 95% CI 0.86 to 1.01; moderate to high-certainty evidence). There were 16 cases of intussusception in 43,629 children after RV5 vaccination and 20 cases in 41,866 children after placebo (RR 0.77, 95% CI 0.41 to 1.45; low-certainty evidence).Rotavac Children vaccinated and followed up the first year of life Rotavac has not been assessed in any RCT in countries with low child mortality. In India, a high-mortality country, Rotavac probably prevents 57% of severe rotavirus diarrhoea cases (RR 0.43, 95% CI 0.30 to 0.60; 6799 participants, moderate-certainty evidence); the trial did not report on severe all-cause diarrhoea at one-year follow-up.Children vaccinated and followed up for two yearsRotavac probably prevents 54% of severe rotavirus diarrhoea cases in India (RR 0.46, 95% CI 0.35 to 0.60; 6541 participants, 1 trial; moderate-certainty evidence), and 16% of severe all-cause diarrhoea cases (RR 0.84, 95% CI 0.71 to 0.98; 6799 participants, 1 trial; moderate-certainty evidence).No increased risk of serious adverse events (SAE) was detected (RR 0.93 95% CI 0.85 to 1.02; moderate-certainty evidence). There were eight cases of intussusception in 5764 children after Rotavac vaccination and three cases in 2818 children after placebo (RR 1.33, 95% CI 0.35 to 5.02; very low-certainty evidence).There was insufficient evidence of an effect on mortality from any rotavirus vaccine (198,381 participants, 44 trials; low- to very low-certainty evidence), as the trials were not powered to detect an effect at this endpoint.
Authors' conclusions: RV1, RV5, and Rotavac prevent episodes of rotavirus diarrhoea. Whilst the relative effect estimate is smaller in high-mortality than in low-mortality countries, there is a greater number of episodes prevented in these settings as the baseline risk is much higher. We found no increased risk of serious adverse events.
Conflict of interest statement
Hanna Bergman: received payment for work on this review from Cochrane Response, an evidence services unit operated by the Cochrane Collaboration. Cochrane Response was contracted by the WHO to produce a systematic review upon which a part of this review update is based (see ‘Sources of support').
Nigel Cunliffe: received research grant support and honoraria for participation in Data Safety Monitoring Boards from GlaxoSmithKline Biologicals.
Femi Pitan: none known.
Nicholas Henschke: received payment for work on this review from Cochrane Response, an evidence services unit operated by the Cochrane Collaboration. Cochrane Response was contracted by the WHO to produce a systematic review upon which a part of this review update is based (see ‘Sources of support').
Karla Soares‐Weiser: has received payment in the past (not for the current update) to conduct this review from the DFID UK via the Effective Health Care Research Programme Consortium (see ‘Sources of support').
Figures














































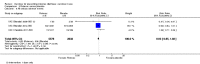









Update of
-
Vaccines for preventing rotavirus diarrhoea: vaccines in use.Cochrane Database Syst Rev. 2012 Nov 14;11:CD008521. doi: 10.1002/14651858.CD008521.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2019 Mar 25;3:CD008521. doi: 10.1002/14651858.CD008521.pub4. PMID: 23152260 Updated.
References
References to studies included in this review
-
- Anh DD, Carlos CC, Thiem DV, Hutagalung Y, Gatchalian S, Bock HL, et al. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 (Rotarix) oral suspension (liquid formulation) when co‐administered with expanded program on immunization (EPI) vaccines in Vietnam and the Philippines in 2006‐2007. Vaccine 2011;29(11):2029‐36. - PubMed
- GlaxoSmithKline[109216‐063]. A phase II, randomized, double‐blind, placebo‐controlled study to evaluate the immunogenicity, reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) liquid vaccine, when given to healthy infants, in Philippines. www.gsk‐studyregister.com/study/3204 (accessed 12 December 2018).
- NCT00432380. Immunogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals' oral live attenuated human rotavirus (HRV) liquid vaccine (GSK 357941A) in healthy infants. clinicaltrials.gov/show/NCT00432380 (first received 7 February 2007).
-
- Anh DD, Carlos CC, Thiem DV, Hutagalung Y, Gatchalian S, Bock HL, et al. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 (Rotarix) oral suspension (liquid formulation) when co‐administered with expanded program on immunization (EPI) vaccines in Vietnam and the Philippines in 2006‐2007. Vaccine 2011;29(11):2029‐36. - PubMed
- Anh DD, Thiem VD, Hutagalung Y, Bock HL, Suryakiran P, Delem A, et al. Immunogenicity, reactogenicity and safety of the oral live attenuated human rotavirus vaccine RIX4414 (Rotarix™) oral suspension (liquid formulation) in Vietnamese Infants. 13th International Congress on Infectious Diseases Abstracts, Poster Presentations. Kuala Lumpur, Malaysia; June 19–22, 2008.
- GlaxoSmithKline[105722‐051]. A phase II, randomized, double‐blind, placebo‐controlled study to evaluate the immunogenicity, reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) liquid vaccine, when given to healthy infants, in Vietnam. www.gsk‐studyregister.com/study/3012 (accessed 12 December 2018).
- NCT00345956. A placebo‐controlled study to evaluate the immunogenicity, reactogenicity and safety of two doses of GSK Bio oral live attenuated human rotavirus (HRV) liquid vaccine, when given to healthy infants, in Vietnam. clinicaltrials.gov/show/NCT00345956 (first received 29 June 2006).
-
- Bernstein DI, Smith VE, Sherwood JR, Schiff GM, Sander DS, DeFeudis D, et al. Safety and immunogenicity of live, attenuated human rotavirus vaccine 89‐12. Vaccine 1998;16(4):381‐7. - PubMed
-
- Bernstein DI, Sack DA, Reisinger K, Rothstein E, Ward RL. Second‐year follow‐up evaluation of live, attenuated human rotavirus vaccine 89‐12 in healthy infants. Journal of Infectious Diseases 2002;186(10):1487‐9. - PubMed
- Bernstein DI, Sack DA, Rothstein E, Reisinger K, Smith VE, O'Sullivan D, et al. Efficacy of live, attenuated, human rotavirus vaccine 89‐12 in infants: a randomised placebo‐controlled trial. Lancet 1999;354(9175):287‐90. - PubMed
-
- Colgate ER, Haque R, Dickson DM, Carmolli MP, Mychaleckyj JC, Nayak U, et al. Delayed dosing of oral rotavirus vaccine demonstrates decreased risk of rotavirus gastroenteritis associated with serum zinc: a randomized controlled trial. Clinical Infectious Diseases 2016;63(5):634‐41. - PubMed
- Kirkpatrick BD, Colgate ER, Mychaleckyj JC, Haque R, Dickson DM, Carmolli MP, et al. The "Performance of Rotavirus and Oral Polio Vaccines in Developing Countries" (PROVIDE) study: description of methods of an interventional study designed to explore complex biologic problems. American Journal of Tropical Medicine and Hygiene 2015;92(4):744‐51. - PMC - PubMed
- Lee B, Dickson DM, DeCamp AC, Colgate ER, Diehl SA, Uddin MI, et al. Histo‐blood group antigen phenotype determines susceptibility to genotype‐specific rotavirus infections and impacts measures of rotavirus vaccine efficacy. Journal of Infectious Diseases 2018;217(9):1399‐407. - PMC - PubMed
- Lee B, Diehl SA, Colgate ER, Dickson DM, Uddin MI, Sharmin S, et al. Lewis antigen and secretor status mediate susceptibility to p‐genotype specific rotavirus infections but do not affect rotavirus vaccine performance among infants in Bangladesh. American Journal of Tropical Medicine and Hygiene 2017;97:226.
- NCT01375647. Exploration of the biologic basis for underperformance of oral polio and rotavirus vaccines in Bangladesh PROVIDE. clinicaltrials.gov/show/NCT01375647 (first received 17 June 2011).
- Rogawski ET, Platts‐Mills JA, Colgate ER, Haque R, Zaman K, Petri WA, et al. Quantifying the impact of natural immunity on rotavirus vaccine efficacy estimates: a clinical trial in Dhaka, Bangladesh (PROVIDE) and a simulation study. Journal of Infectious Diseases 2018;217(6):861‐8. - PMC - PubMed
References to studies excluded from this review
-
- Armah GE, Kapikian AZ, Vesikari T, Cunliffe N, Jacobson RM, Burlington DB, et al. Efficacy, immunogenicity, and safety of two doses of a tetravalent rotavirus vaccine RRV‐TV in Ghana with the first dose administered during the neonatal period. Journal of Infectious Diseases 2013;208(3):423‐31. - PMC - PubMed
-
- ACTRN12611001212943. RV3‐BB rotavirus vaccine phase IIa clinical trial of immunogenicity and safety. anzctr.org.au/ACTRN12611001212943.aspx (first received 24 November 2011).
- Bines JE, Danchin M, Jackson P, Handley A, Watts E, Lee KJ, et al. RV3 Rotavirus Vaccine Program. Safety and immunogenicity of RV3‐BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double‐blind, placebo‐controlled trial. Lancet Infectious Diseases 2015;15(12):1389‐97. - PubMed
-
- Bucher A, Rivara G, Briceno D, Huicho L. [Use of a rapid rotavirus test in prescription of antibiotics in acute diarrhea in pediatrics: an observational, randomized, controlled study]. Revista de Gastroenterologia del Peru 2012;32(1):11‐5. - PubMed
References to ongoing studies
-
- ACTRN12610000525088. A Phase 1 double‐blind, randomized study to compare the safety, tolerability and immunogenicity of oral RV3‐BB rotavirus vaccine and placebo in infants, children and male adults. anzctr.org.au/ACTRN12610000525088.aspx (first received 22 June 2010).
-
- CTRI/2015/07/006034. Clinical trial on rotavirus vaccine to check consistency of different lots of vaccines manufactured and to check vaccine interference with other childhood vaccines given under universal immunization program in India. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=10177&EncHid=&am... (first received 20 July 2015).
-
- CTRI/2015/12/006428. Randomized open label study to compare immunogenicity and safety of ROTAVAC® and ROTARIX® rotavirus vaccine. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=13227&EncHid=&am... (first received 8 December 2015).
-
- NCT01061658. Phase I/II, randomized, double‐blind, placebo‐controlled, dosage selection (10e5.5 or 10e6.25 FFU of each constituent serotype per 0.5 mL) study to evaluate the safety, tolerability, and immunogenicity of a 3‐dose Series of live attenuated tetravalent (G1‐G4) bovine‐human reassortant rotavirus vaccine [BRV‐TV] administered to healthy Indian infants. clinicaltrials.gov/show/NCT01061658 (accessed 3 February 2010).
-
- NCT02153866. The safety and immunogenicity study of rotavirus vaccine simultaneously vaccinated with MR or MMR vaccine. clinicaltrials.gov/ct2/show/NCT02153866 (first received 3 June 2014).
Additional references
-
- Bines JE. Rotavirus vaccines and intussusception risk. Current Opinion in Gastroenterology 2005;21(1):20‐5. - PubMed
-
- Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: Post‐marketing surveillance in the National Immunization Program in Australia. Vaccine 2011;29(16):3061‐66. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

