Joint Encoding of Auditory Timing and Location in Visual Cortex
- PMID: 30912728
- PMCID: PMC6551606
- DOI: 10.1162/jocn_a_01399
Joint Encoding of Auditory Timing and Location in Visual Cortex
Abstract
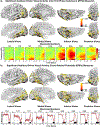
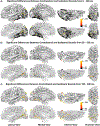
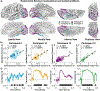
Co-occurring sounds can facilitate perception of spatially and temporally correspondent visual events. Separate lines of research have identified two putatively distinct neural mechanisms underlying two types of crossmodal facilitations: Whereas crossmodal phase resetting is thought to underlie enhancements based on temporal correspondences, lateralized occipital evoked potentials (ERPs) are thought to reflect enhancements based on spatial correspondences. Here, we sought to clarify the relationship between these two effects to assess whether they reflect two distinct mechanisms or, rather, two facets of the same underlying process. To identify the neural generators of each effect, we examined crossmodal responses to lateralized sounds in visually responsive cortex of 22 patients using electrocorticographic recordings. Auditory-driven phase reset and ERP responses in visual cortex displayed similar topography, revealing significant activity in pericalcarine, inferior occipital-temporal, and posterior parietal cortex, with maximal activity in lateral occipitotemporal cortex (potentially V5/hMT+). Laterality effects showed similar but less widespread topography. To test whether lateralized and nonlateralized components of crossmodal ERPs emerged from common or distinct neural generators, we compared responses throughout visual cortex. Visual electrodes responded to both contralateral and ipsilateral sounds with a contralateral bias, suggesting that previously observed laterality effects do not emerge from a distinct neural generator but rather reflect laterality-biased responses in the same neural populations that produce phase-resetting responses. These results suggest that crossmodal phase reset and ERP responses previously found to reflect spatial and temporal facilitation in visual cortex may reflect the same underlying mechanism. We propose a new unified model to account for these and previous results.
Figures
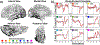



References
-
- Alho K, Medvedev SV, Pakhomov SV, Roudas MS, Tervaniemi M, Reinikainen K, … Näätänen R (1999). Selective tuning of the left and right auditory cortices during spatially directed attention. Cognitive Brain Research, 7(3), 335–341. - PubMed
-
- Bair W, & O’keefe LP (1998). The influence of fixational eye movements on the response of neurons in area MT of the macaque. Visual Neuroscience, 15(4), 779–786. - PubMed
-
- Banerjee S, Snyder AC, Molholm S, & Foxe JJ (2011). Oscillatory alpha-band mechanisms and the deployment of spatial attention to anticipated auditory and visual target locations: supramodal or sensory-specific control mechanisms? Journal of Neuroscience, 31(27), 9923–9932. 10.1523/JNEUROSCI.4660-10.2011/ - DOI - PMC - PubMed
-
- Brainard DH (1997). The Psychophysics Toolbox. Spatial Vision, 10(4), 433–436. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

