Intramyocardial Injection of Mesenchymal Precursor Cells and Successful Temporary Weaning From Left Ventricular Assist Device Support in Patients With Advanced Heart Failure: A Randomized Clinical Trial
- PMID: 30912838
- PMCID: PMC6439694
- DOI: 10.1001/jama.2019.2341
Intramyocardial Injection of Mesenchymal Precursor Cells and Successful Temporary Weaning From Left Ventricular Assist Device Support in Patients With Advanced Heart Failure: A Randomized Clinical Trial
Erratum in
-
Incorrect Unit of Measure.JAMA. 2019 Sep 3;322(9):895. doi: 10.1001/jama.2019.12207. JAMA. 2019. PMID: 31479120 Free PMC article. No abstract available.
Abstract
Importance: Left ventricular assist device (LVAD) therapy improves myocardial function, but few patients recover sufficiently for explant, which has focused attention on stem cells to augment cardiac recovery.
Objective: To assess efficacy and adverse effects of intramyocardial injections of mesenchymal precursor cells (MPCs) during LVAD implant.
Design, setting, and participants: A randomized phase 2 clinical trial involving patients with advanced heart failure, undergoing LVAD implant, at 19 North American centers (July 2015-August 2017). The 1-year follow-up ended August 2018.
Interventions: Intramyocardial injections of 150 million allogeneic MPCs or cryoprotective medium as a sham treatment in a 2:1 ratio (n = 106 vs n = 53).
Main outcomes and measures: The primary efficacy end point was the proportion of successful temporary weans (of 3 planned assessments) from LVAD support within 6 months of randomization. This end point was assessed using a Bayesian analysis with a predefined threshold of a posterior probability of 80% to indicate success. The 1-year primary safety end point was the incidence of intervention-related adverse events (myocarditis, myocardial rupture, neoplasm, hypersensitivity reactions, and immune sensitization). Secondary end points included readmissions and adverse events at 6 months and 1-year survival.
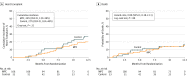
Results: Of 159 patients (mean age, 56 years; 11.3% women), 155 (97.5%) completed 1-year of follow-up. The posterior probability that MPCs increased the likelihood of successful weaning was 69%; below the predefined threshold for success. The mean proportion of successful temporary weaning from LVAD support over 6 months was 61% in the MPC group and 58% in the control group (rate ratio [RR], 1.08; 95% CI, 0.83-1.41; P = .55). No patient experienced a primary safety end point. Of 10 prespecified secondary end points reported, 9 did not reach statistical significance. One-year mortality was not significantly different between the MPC group and the control group (14.2% vs 15.1%; hazard ratio [HR], 0.89; 95%, CI, 0.38-2.11; P = .80). The rate of serious adverse events was not significantly different between groups (70.9 vs 78.7 per 100 patient-months; difference, -7.89; 95% CI, -39.95 to 24.17; P = .63) nor was the rate of readmissions (0.68 vs 0.75 per 100 patient-months; difference, -0.07; 95% CI, -0.41 to 0.27; P = .68).
Conclusions and relevance: Among patients with advanced heart failure, intramyocardial injections of mesenchymal precursor cells, compared with injections of a cryoprotective medium as sham treatment, did not improve successful temporary weaning from left ventricular assist device support at 6 months. The findings do not support the use of intramyocardial mesenchymal stem cells to promote cardiac recovery as measured by temporary weaning from device support.
Trial registration: clinicaltrials.gov Identifier: NCT02362646.
Conflict of interest statement
Figures


Comment in
-
Stem Cell Therapy for Heart Failure: An Unfulfilled Promise?JAMA. 2019 Mar 26;321(12):1186-1187. doi: 10.1001/jama.2019.2617. JAMA. 2019. PMID: 30912818 No abstract available.
-
Can stem cell therapy increase the rate of myocardial recovery in left ventricular assist device-supported advanced heart failure patients?-current data and future perspectives.Ann Transl Med. 2019 Nov;7(22):613. doi: 10.21037/atm.2019.10.60. Ann Transl Med. 2019. PMID: 31930014 Free PMC article. No abstract available.
References
-
- Suncion VY, Ghersin E, Fishman JE, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally? an analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res. 2014;114(8):1292-1301. doi: 10.1161/CIRCRESAHA.114.302854 - DOI - PMC - PubMed
-
- Karantalis V, DiFede DL, Gerstenblith G, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114(8):1302-1310. doi: 10.1161/CIRCRESAHA.114.303180 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

