Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression
- PMID: 30913039
- PMCID: PMC6546453
- DOI: 10.1172/JCI124218
Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression
Abstract
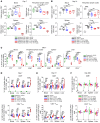
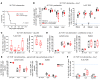
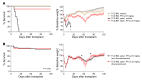
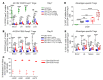
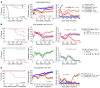
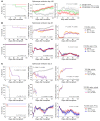
Post-transplantation cyclophosphamide (PTCy) recently has had a marked impact on human allogeneic hematopoietic cell transplantation (HCT). Yet, our understanding of how PTCy prevents graft-versus-host disease (GVHD) largely has been extrapolated from major histocompatibility complex (MHC)-matched murine skin allografting models that were highly contextual in their efficacy. Herein, we developed a T-cell-replete, MHC-haploidentical, murine HCT model (B6C3F1→B6D2F1) to test the putative underlying mechanisms: alloreactive T-cell elimination, alloreactive T-cell intrathymic clonal deletion, and suppressor T-cell induction. In this model and confirmed in four others, PTCy did not eliminate alloreactive T cells identified using either specific Vβs or the 2C or 4C T-cell receptors. Furthermore, the thymus was not necessary for PTCy's efficacy. Rather, PTCy induced alloreactive T-cell functional impairment which was supported by highly active suppressive mechanisms established within one day after PTCy that were sufficient to prevent new donor T cells from causing GVHD. These suppressive mechanisms included the rapid, preferential recovery of CD4+CD25+Foxp3+ regulatory T cells, including those that were alloantigen-specific, which served an increasingly critical function over time. Our results prompt a paradigm-shift in our mechanistic understanding of PTCy. These results have direct clinical implications for understanding tolerance induction and for rationally developing novel strategies to improve patient outcomes.
Keywords: Bone marrow transplantation; Immunology; T cells; Tolerance; Transplantation.
Conflict of interest statement
Figures




Comment in
-
Mechanism of action of posttransplantation cyclophosphamide: more than meets the eye.J Clin Invest. 2019 May 6;129(6):2189-2191. doi: 10.1172/JCI128710. eCollection 2019 May 6. J Clin Invest. 2019. PMID: 31063990 Free PMC article.
References
-
- McCurdy SR, et al. Grade II acute graft-versus-host disease and higher nucleated cell graft dose improve progression-free survival after HLA-haploidentical transplant with post-transplant cyclophosphamide. Biol Blood Marrow Transplant. 2018;24(2):343–352. doi: 10.1016/j.bbmt.2017.10.023. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

