Autoantibodies are present before the clinical diagnosis of systemic sclerosis
- PMID: 30913258
- PMCID: PMC6435159
- DOI: 10.1371/journal.pone.0214202
Autoantibodies are present before the clinical diagnosis of systemic sclerosis
Abstract
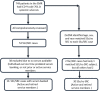
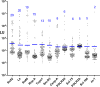
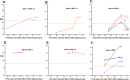
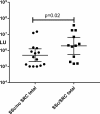
Systemic sclerosis (SSc) is a heterogeneous autoimmune disorder associated with vascular dysfunction and fibrotic changes in the skin, vasculature and internal organs. Although serologic abnormalities are an important diagnostic tool for SSc, little is known about whether autoantibodies precede clinical diagnosis. Here we investigated the presence of autoantibodies before SSc diagnosis and assessed whether certain autoantibodies might associate with the future onset of scleroderma renal crisis (SRC), a potentially fatal complication of the disease. Using the Department of Defense Serum Repository, autoantibodies were analyzed from archived, prospectively collected, longitudinal serum samples from sixteen individuals with SRC (SSc/SRC) and thirty cases of SSc without SRC (SSc/no SRC), matched for age, sex, and race. Seventy five percent (12/16) of the SSc/SRC and 40% (12/30) of the SSc/no SRC were seropositive for at least one autoantibody prior to clinical diagnosis (up to 27.1 years earlier, mean = -7.4 years). Although both disease groups demonstrated a heterogeneous immunoreactivity profile against the autoantigen panel, the SSc/SRC subjects showed two enriched clusters with one featuring elevated levels of autoantibodies against Ro52 and/or Ro60 and another with high levels of immunoreactivity against the RNA polymerase complex. Consistent with larger spectrum of immunoreactivity and the elevated levels of autoantibodies in SSc/SRC, the total response against the autoantigen panel from the last time point of the seropositive subjects revealed that the SSc/SRC cohort harbored higher antibody levels (p = 0.02) compared to SSc/no SRC. Overall, our findings demonstrate that relevant seropositive autoantibodies often precede the clinical diagnosis of SSc/no SRC and SSc/SRC.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr., et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202–5. Epub 1988/02/01. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

