Independent Roles of Estrogen Deficiency and Cellular Senescence in the Pathogenesis of Osteoporosis: Evidence in Young Adult Mice and Older Humans
- PMID: 30913313
- PMCID: PMC6697189
- DOI: 10.1002/jbmr.3729
Independent Roles of Estrogen Deficiency and Cellular Senescence in the Pathogenesis of Osteoporosis: Evidence in Young Adult Mice and Older Humans
Abstract
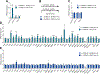
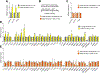
Estrogen deficiency is a seminal mechanism in the pathogenesis of osteoporosis. Mounting evidence, however, establishes that cellular senescence, a fundamental mechanism that drives multiple age-related diseases, also causes osteoporosis. Recently, we systematically identified an accumulation of senescent cells, characterized by increased p16Ink4a and p21Cip1 levels and development of a senescence-associated secretory phenotype (SASP), in mouse bone/marrow and human bone with aging. We then demonstrated that elimination of senescent cells prevented age-related bone loss using multiple approaches, eg, treating old mice expressing a "suicide" transgene, INK-ATTAC, with AP20187 to induce apoptosis of p16Ink4a -senescent cells or periodically treating old wild-type mice with "senolytics," ie, drugs that eliminate senescent cells. Here, we investigate a possible role for estrogen in the regulation of cellular senescence using multiple approaches. First, sex steroid deficiency 2 months after ovariectomy (OVX, n = 15) or orchidectomy (ORCH, n = 15) versus sham surgery (SHAM, n = 15/sex) in young adult (4-month-old) wild-type mice did not alter senescence biomarkers or induce a SASP in bone. Next, in elderly postmenopausal women, 3 weeks of estrogen therapy (n = 10; 74 ± 5 years) compared with no treatment (n = 10; 78 ± 5 years) did not alter senescence biomarkers or the SASP in human bone biopsies. Finally, young adult (4-month-old) female INK-ATTAC mice were randomized (n = 17/group) to SHAM+Vehicle, OVX+Vehicle, or OVX+AP20187 for 2 months. As anticipated, OVX+Vehicle caused significant trabecular/cortical bone loss compared with SHAM+Vehicle. However, treatment with AP20187, which eliminates senescent cells in INK-ATTAC mice, did not rescue the OVX-induced bone loss or alter senescence biomarkers. Collectively, our data establish independent roles of estrogen deficiency and cellular senescence in the pathogenesis of osteoporosis, which has important implications for testing novel senolytics for skeletal efficacy, as these drugs will need to be evaluated in preclinical models of aging as opposed to the current FDA model of prevention of OVX-induced bone loss. © 2019 American Society for Bone and Mineral Research.
Keywords: AGING; ANIMAL MODELS; BONE; ESTROGEN THERAPY; OSTEOCYTE; OSTEOPOROSIS; SEX STEROIDS.
© 2019 American Society for Bone and Mineral Research.
Conflict of interest statement
Figures



References
-
- Albright F Post-menopausal osteoporosis. Trans Assoc Am Physicians 1940;55:298–305.
-
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. Jama 2002;288(3):321–33. - PubMed
-
- Parente L, Uyehara C, Larsen W, Whitcomb B, Farley J. Long-term impact of the women’s health initiative on HRT. Arch Gynecol Obstet 2008;277(3):219–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG049182/AG/NIA NIH HHS/United States
- AG013925/AG/NIA NIH HHS/United States
- AR068275/AR/NIAMS NIH HHS/United States
- R37 AG013925/AG/NIA NIH HHS/United States
- R01 AG048792/AG/NIA NIH HHS/United States
- R21 AG049182/AG/NIA NIH HHS/United States
- P01 AG062413/AG/NIA NIH HHS/United States
- AR070241/AR/NIAMS NIH HHS/United States
- K01 AR070241/AR/NIAMS NIH HHS/United States
- P01 AG004875/AG/NIA NIH HHS/United States
- AG048792/AG/NIA NIH HHS/United States
- R01 AG013925/AG/NIA NIH HHS/United States
- AG004875/AG/NIA NIH HHS/United States
- R01 AR068275/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

