A randomised controlled trial of matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDITOF-MS) versus conventional microbiological methods for identifying pathogens: Impact on optimal antimicrobial therapy of invasive bacterial and fungal infections in Vietnam
- PMID: 30914268
- PMCID: PMC6529875
- DOI: 10.1016/j.jinf.2019.03.010
A randomised controlled trial of matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDITOF-MS) versus conventional microbiological methods for identifying pathogens: Impact on optimal antimicrobial therapy of invasive bacterial and fungal infections in Vietnam
Abstract
Objectives: We assessed the impact of MALDITOF-MS on the timeliness of optimal antimicrobial therapy through a parallel-arm randomised controlled trial in two hospitals in Vietnam.
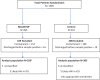
Methods: We recruited patients with a pathogen (bacterial or fungal) cultured from a normally sterile sample. Samples were randomly assigned (1:1) to identification by MALDITOF-MS or conventional diagnostics. The primary outcome was the proportion on optimal antimicrobial therapy within 24 h of positive culture, determined by a blinded independent review committee. Trial registered at ClinicalTrials.gov (NCT02306330).
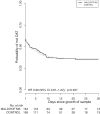
Results: Among 1005 randomised patients, pathogens were isolated from 628 (326 intervention, 302 control), with 377 excluded as likely contaminants or discharged/died before positive culture. Most isolates were cultured from blood (421/628, 67.0%). The proportion receiving optimal antimicrobial therapy within 24 h (the primary outcome) or 48 h of growth was not significantly different between MALDITOF-MS and control arms (135/326, 41.4% vs 120/302, 39.7%; Adjusted Odds ration (AOR) 1.17, p = 0.40 and 151/326, 46.3% vs 141/302, 46.7%; AOR 1.05 p = 0.79, respectively).
Conclusions: MALDITOF-MS, in the absence of an antimicrobial stewardship programme, did not improve the proportion on optimal antimicrobial therapy at 24 or 48 h after first growth in a lower-middle income setting with high rates of antibiotic resistance.
Keywords: Antibacterial agents; Bacteraemia; Matrix-assisted laser desorption-ionization mass spectrometry; Microbiological techniques; Vietnam.
Copyright © 2019. Published by Elsevier Ltd.
Figures
References
-
- Petti C.A., Polage C.R., Quinn T.C., Ronald A.R., Sande M.A. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42(3):377–382. - PubMed
-
- Yao K., McKinney B., Murphy A., Rotz P., Wafula W., Sendagire H. Improving quality management systems of laboratories in developing countries: an innovative training approach to accelerate laboratory accreditation. Am J Clin Pathol. 2010;134(3):401–409. - PubMed
-
- Kinh N.V., Wertheim H.F.L., Thwaites G.E., Khue L.N., Thai C.H., Khoa N.T. Developing an antimicrobial resistance reference laboratory and surveillance programme in Vietnam. Lancet Glob Health. 2017;5(12):e1186–e11e7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical