Impaired human hematopoiesis due to a cryptic intronic GATA1 splicing mutation
- PMID: 30914438
- PMCID: PMC6504223
- DOI: 10.1084/jem.20181625
Impaired human hematopoiesis due to a cryptic intronic GATA1 splicing mutation
Abstract
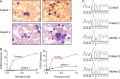
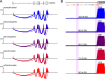
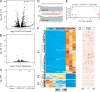
Studies of allelic variation underlying genetic blood disorders have provided important insights into human hematopoiesis. Most often, the identified pathogenic mutations result in loss-of-function or missense changes. However, assessing the pathogenicity of noncoding variants can be challenging. Here, we characterize two unrelated patients with a distinct presentation of dyserythropoietic anemia and other impairments in hematopoiesis associated with an intronic mutation in GATA1 that is 24 nucleotides upstream of the canonical splice acceptor site. Functional studies demonstrate that this single-nucleotide alteration leads to reduced canonical splicing and increased use of an alternative splice acceptor site that causes a partial intron retention event. The resultant altered GATA1 contains a five-amino acid insertion at the C-terminus of the C-terminal zinc finger and has no observable activity. Collectively, our results demonstrate how altered splicing of GATA1, which reduces levels of the normal form of this master transcription factor, can result in distinct changes in human hematopoiesis.
© 2019 Abdulhay et al.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

